Mineral, naturally occurring homogeneous solid with a definite chemical composition and a highly ordered atomic arrangement; it is usually formed by inorganic processes. There are several thousand known mineral species, about 100 of which constitute the major mineral components of rocks; these are the so-called rock-forming minerals.

azuriteAzurite, also called chessylite, is a basic copper carbonate ordinarily found with malachite in the oxidized zone of copper lodes.
Definition
A mineral, which by definition must be formed through natural processes, is distinct from the synthetic equivalents produced in the laboratory. Artificial versions of minerals, including emeralds, sapphires, diamonds, and other valuable gemstones, are regularly produced in industrial and research facilities and are often nearly identical to their natural counterparts.
By its definition as a homogeneous solid, a mineral is composed of a single solid substance of uniform composition that cannot be physically separated into simpler chemical compounds. Homogeneity is determined relative to the scale on which it is defined. A specimen that appears homogeneous to the unaided eye, for example, may reveal several mineral components under a microscope or upon exposure to X-ray diffraction techniques. Most rocks are composed of several different minerals; e.g., granite consists of feldspar, quartz, mica, and amphibole. In addition, gases and liquids are excluded by a strict interpretation of the above definition of a mineral. Ice, the solid state of water (H2O), is considered a mineral, but liquid water is not; liquid mercury, though sometimes found in mercury ore deposits, is not classified as a mineral either. Such substances that resemble minerals in chemistry and occurrence are dubbed mineraloids and are included in the general domain of mineralogy.

Since a mineral has a definite composition, it can be expressed by a specific chemical formula. Quartz (silicon dioxide), for instance, is rendered as SiO2, because the elements silicon (Si) and oxygen (O) are its only constituents and they invariably appear in a 1:2 ratio. The chemical makeup of most minerals is not as well defined as that of quartz, which is a pure substance. Siderite, for example, does not always occur as pure iron carbonate (FeCO3); magnesium (Mg), manganese (Mn), and, to a limited extent, calcium (Ca) may sometimes substitute for the iron. Since the amount of the replacement may vary, the composition of siderite is not fixed and ranges between certain limits, although the ratio of the metal cation to the anionic group remains fixed at 1:1. Its chemical makeup may be expressed by the general formula (Fe, Mn, Mg, Ca)CO3, which reflects the variability of the metal content.

Trigonal systemTrigonal (rhombohedral) crystals of quartz.
Minerals display a highly ordered internal atomic structure that has a regular geometric form. Because of this feature, minerals are classified as crystalline solids. Under favourable conditions, crystalline materials may express their ordered internal framework by a well-developed external form, often referred to as crystal form or morphology. Solids that exhibit no such ordered internal arrangement are termed amorphous. Many amorphous natural solids, such as glass, are categorized as mineraloids.

pyriteSchematic representation of the structure of pyrite (FeS2) as based on a cubic array of ferrous iron cations (Fe2+) and sulfur anions (S−).
Traditionally, minerals have been described as resulting exclusively from inorganic processes; however, current mineralogic practice often includes as minerals those compounds that are organically produced but satisfy all other mineral requirements. Aragonite (CaCO3) is an example of an inorganically formed mineral that also has an organically produced, yet otherwise identical, counterpart; the shell (and the pearl, if it is present) of an oyster is composed to a large extent of organically formed aragonite. Minerals also are produced by the human body: hydroxylapatite [Ca5(PO4)3(OH)] is the chief component of bones and teeth, and calculi are concretions of mineral substances found in the urinary system.
Nomenclature
While minerals are classified in a logical manner according to their major anionic (negatively charged) chemical constituents into groups such as oxides, silicates, and nitrates, they are named in a far less scientific or consistent way. Names may be assigned to reflect a physical or chemical property, such as colour, or they may be derived from various subjects deemed appropriate, such as, for example, a locality, public figure, or mineralogist. Some examples of mineral names and their derivations follow: albite (NaAlSi3O8) is from the Latin word (albus) for “white” in reference to its colour; goethite (FeO ∙ OH) is in honour of Johann Wolfgang von Goethe, the German poet; manganite (MnO ∙ OH) reflects the mineral’s composition; franklinite (ZnFe2O4) is named after Franklin, New Jersey, U.S., the site of its occurrence as the dominant ore mineral for zinc (Zn); and sillimanite (Al2SiO4) is in honour of the American chemist Benjamin Silliman. Since 1960 the Commission on New Minerals and Mineral Names of the International Mineralogical Association has reviewed descriptions of new minerals and proposals for new mineral names and has attempted to remove inconsistencies. Any new mineral name must be approved by this committee, and the type material is usually stored in a museum or university collection.
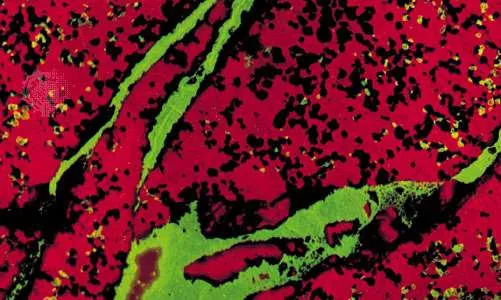
franklinite, willemite, and calciteSpecimen showing bright fluorescent colour resulting from ultraviolet irradiation of calcite (calcium carbonate; red fluorescence) with grains of franklinite (a zinc, manganese, and iron oxide; black coloration), which is nonfluorescent. Veins and grains of willemite (a zinc silicate; green fluorescence) also appear.


