What is a mole?
For our purposes we will say that a mole is a certain amount of material corresponding to a specified number of molecules, atoms, electrons, or other specified types of particles. In the SI system a mole (which we will call a gram mole to avoid confusing units) is composed of 6.022 × 10 23 (Avogadro’s number) molecules.
However, for convenience in calculations and for clarity, we will make use of other specifications for moles such as the pound mole (lb mol, composed of 6.022 × 10 23 × 453.6) molecules, the kg mol (kilomole, kmol, composed of 1000 moles), and so on. You will find that such nonconforming (to SI) definitions of the amount of material will help avoid excess details in many calculations.
What would a metric ton mole of molecules consist of? One important calculation at which you should become skilled is to convert the number of moles to mass and the mass to moles. To do this you make use of the molecular weight—the mass per mole:
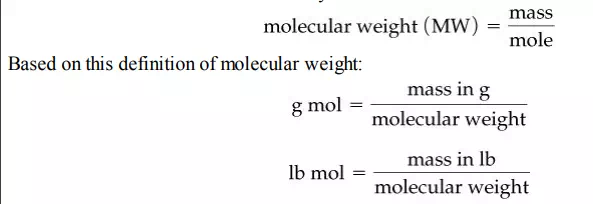
Therefore, from the definition of the molecular weight, you can calculate the mass knowing the number of moles or the number of moles knowing the mass. For historical reasons, the terms atomic weight and molecular weight are usually used instead of the more accurate terms atomic mass and molecular mass.
Use of Molecular Weights to Convert Mass to Moles
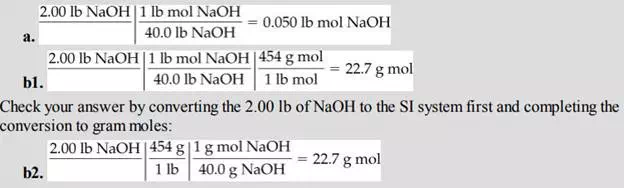
If a bucket holds 2.00 lb of NaOH:
a. How many pound moles of NaOH does it contain?
b. How many gram moles of NaOH does it contain?
Solution
You can convert pounds to pound moles, and then convert the values to the SI system of units. Look up the molecular weight of NaOH, or calculate it from the atomic weights. (It is 40.0.) Note that the molecular weight is used as a conversion factor in this calculation:
Values of the molecular weights (relative molar masses) are built up from the values of atomic weights based on a scale of the relative masses of the elements. The atomic weight of an element is the mass of an atom based on the scale that assigns a mass of exactly 12 to the carbon isotope 12C. The value 12 is selected in this case because an atom of carbon 12 contains 6 protons and 6 neutrons for a total molecular weight of 12


