In ancient times, counterfeit gold objects were identified by comparing the ratio of the weight to the volume of water displaced by the object, which is a way to measure the density of the material of the object, to that of an object known to be made of gold. A striking example of quick thinking by an engineer who made use of the concept of density was reported by P. K. N. Paniker in the June 15, 1970, issue of Chemical Engineering:
The bottom outlet nozzle of a full lube-oil storage tank kept at a temperature of about 80°C suddenly sprang a gushing leak as the nozzle flange became loose. Because of the high temperature of the oil, it was impossible for anyone to go near the tank and repair the leak to prevent further loss. After a moment of anxiety, we noticed that the engineer in charge rushed to his office to summon fire department personnel and instruct them to run a hose from the nearest fire hydrant to the top of the storage tank.
Within minutes, what gushed out from the leak was hot water instead of valuable oil. Some time later, as the entering cold water lowered the oil temperature, it was possible to make repairs. Density (we use the Greek symbol ρ) is the ratio of mass per unit volume such as kg/m3 or lb/ft 3 :
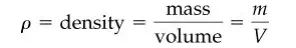
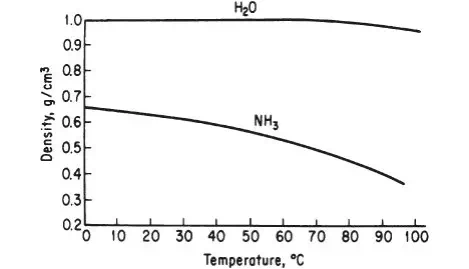
Density has both a numerical value and units. Densities for liquids and solids do not change significantly at ordinary conditions with pressure, but they can change significantly with temperature for certain compounds if the temperature change is large enough, as shown in Figure 2.1. Note that between 0°C and 70°C, the density of water is relatively constant at 1.0 g/cm3 . On the other hand, for the same temperature range, the density of NH3 changes by approximately 30%. Usually we will ignore the effect of temperature on liquid density unless the density of the material is especially sensitive to temperature or the change in the temperature is particularly large