The isomerisation process involves the transformation of one molecular structure into another (isomer) whose component atoms are the same but arranged in a different geometrical structure. Since isomers may differ greatly in physical and chemical properties, isomerisation offers the possibility of converting less desirable compounds into isomers with desirable properties, in particular to convert n-paraffins into iso-paraffins, thereby increasing the octane of the hydrocarbon stream. The main fields of application of isomerisation are:
ISOMERISATION of normal butane into isobutane ISOMERISATION of pentanes and hexanes into higher- branched isomers
Since branched isomers have a higher antiknock quality than the corresponding linear paraffins, this form of isomerisation is important for the production of motor fuels.
In addition to the above applications, isomerisation is applied for the conversion of ortho-xylene and meta- xylene into para- xylene, used for the manufacturing of polester fibres.
Isomerisation of low molecular weight paraffins has been commercially applied for many years. After extensive laboratory work had been carried out during the 1930s, World War 2 prompted the development of the laboratory processes into full- scale commercial units in order to meet the demand for isobutane necessary for the manufacture of large amounts of alkylate. While the first butane isomerisation unit went on stream in late 1941, by the end of the war nearly 40 butane isomerisation units were in operation in the USA and the Caribbean. Two pentane and two light naphtha isomerisation units also came on stream towards the end of the war to provide an additional source of blending aviation gasoline.
Though butane isomerisation has maintained its importance, present day interest isomerisation is specially focussed on the upgrading of fractions containing C5 Pentane and C6 Hexane for use as motor gasoline components. This application has been prompted by the world drive to remove the lead additives gradually from motor gasoline in order to reduce air pollution. The octane loss caused by the removal or reduction of lead antiknock additives can be compensated for by isomerisation of pentane/hexane paraffin fraction of the light gasoline fractions.
 |
| Figure 5: Typical Isomerization Process |
Isomerisation technology has also improved substantially due to the hard work of many technologist. In order to achieve the low temperature necessary to obtain an acceptable yield of isomers, the Catalyst systems used in the early units were based on aluminium chloride in some form. These catalyst systems, however, had the drawback of being highly corrosive and difficult to handle. In recent years, catalyst of a different type have come in use. These are solid catalysts consisting of a support having an acidic carrier and a hydrogenation function, frequently a noble metal. Modern isomerisation units utilise these dual- function catalysts and operate in the vapour phase and the presence of hydogen. For these reasons, these process are called hydro- isomerisation processes.
The first hydro- isomerisation unit was introduced in 1953 by UOP, followed in 1965 by the first BP one, while in 1970 the first Shell hydro-isomerisation (HYSOMER) unit was started up. At present the following hydro-isomerisation processes are commercially available:
● UOP BUTAMER for butane isomerisation
● UOP PENEX for pentane/hexane isomerisation
● BP C4 isomerisation for butane isomerisation
● BP C5/C6 isomerisation for pentane/hexane isomerisation
● SHELL Hysomer for pentane/hexane isomerisation
All these processes takes place in the vapour phase on a fixed bed catalyst containing platinum on a solid carrier.
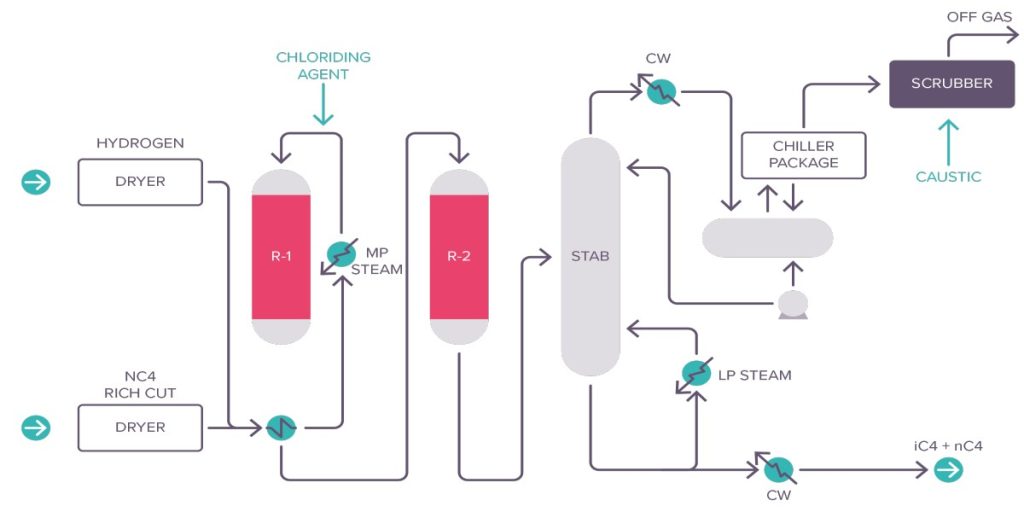
As an example, the Shell Hysomer process will be briefly described. The liquid feedstock is pentane/hexane from light naphtha. naphtha splitters are widely used to split light naphtha, heavy naphtha and also LPG. The light naphtha (C5/C6) is combined with the recycle gas/ fresh gas mixture. The resultant combined reactor feed is routed to a feed/ effluent heat exchanger, where it is heated and completely vaporised by the effluent of the reactor. The vapourised combined reactor feed is further heated to the desired reactor inlet temperature in the reactor charge heater. The hot charge enters the Hysomer reactor at the top and flows downwards through the catalyst bed, where a portion of normal and mono- branched paraffins is converted into higher branched (high octane) components. Temperature rise from the heat of reaction release is controlled by a cold quench gas injection into the reactor. Reactor effluent is cooled and subsequently separated in the product separator into two streams: a liquid product (isomerate) and a recycle gas stream returning to the reactor via the recycle gas compressor.
The catalyst is a dual function catalyst consisting of platinum on a zeolite basis, highly stable and regenerable.
Temperatures and pressure vary in a range of 230 – 285 0C and 13-30 bar, C5/C6 content in product relative to that in feed is 97% or better, and octane upgrading ranges between 8 and 10 points, depending on feedstock quality. The Hysomer process can be integrated with catalytic reformer, resulting in substantial equipment savings, or with iso-normal separation processes which allows for a complete conversion of pentane/hexane mixtures into isoparaffin mixtures. An interesting application in this field is the total isomerisation process (TIP) in which the isomerisation is completely integrated with a Union Carbide molecular sieve separation process or the naphtha IsoSiv Process by UOP.