We all sweat at some time or other, e.g. after running hard, living We need the salt in sweat to decrease the water’s surface tension in order to speed up the evaporation process (we feel cooler more quickly). The oils in sweat prevents the skin from drying out, which would make it susceptible to sunburn.
Evaporation is also called ‘vaporization’. It is a thermodynamic process, because energy is transferred. in a hot climate or perhaps during an illness when our temperature is raised due to an infection (which is why we sometimes say, we have ‘got a temperature’). Producing sweat is one of the body’s natural ways of cooling itself, and it operates as follows.
Sweat is an aqueous solution of salt and natural oils, and is secreted by glands just below the surface of the skin. The glands generate this mixture whenever the body feels too hot. Every time air moves over a sweaty limb, from a mechanical fan or natural breeze, the skin feels cooler following evaporation of water from the sweat. When we say the water evaporates when a breeze blows, we mean it undergoes a phase transition from liquid to vapour, i.e. a phase transition proceeding in the opposite direction to that in the previous example, so Equation (3.2) occurs backwards. When we consider the internal energy changes, we see U(final) = U(water, g) and U(initial) = U(water, l), so the final state of the water here is more energetic than was its initial state. Figure 3.2 shows a schematic representation of the energy change involved.
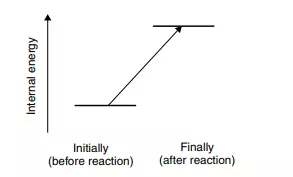
The definition of U in Equation (3.1) is U = U(final) − U(initial), so the value of U(evaporation) is obtained as U(water, g) − U(water, l). We already know that the final state of the water is more energetic than its initial state, so the value of U is positive. We say such a process is endothermic. We feel cooler when sweating because the skin loses energy by transferring it to the water on its surface, which then evaporates. This process of water evaporation (sweating) is endothermic because energy passes from the skin to the water, and a body containing less energy has a lower temperature, which is why we feel cooler.
State functions
Sometimes we feel hot even when sweating, particularly in a humid environment like a beach by the sea on a hot day. Two processes occur in tandem on the skin: evaporation (liquid water → gaseous water) and condensation (gaseous water → liquid water). It is quite possible that the same water condenses on our face as evaporated earlier. In effect, then, a cycle of ‘liquid → gas → liquid’ occurs. The two halves of this cycle operate in opposite senses, since both exo- and endo-thermic processes occur simultaneously. The net change in energy is, therefore, negligible, and we feel no cooler.
These two examples of energy change involve water. The only difference between them is the direction of change, and hence the sign of U. But these two factors are related. If we were to condense exactly 1 mol of steam then the amount of energy released into the skin would be 40 700 J. The change in internal energy U (ignoring volume changes) is negative because energy is given out during the condensation process, so U = −40 700 J
Comments are closed