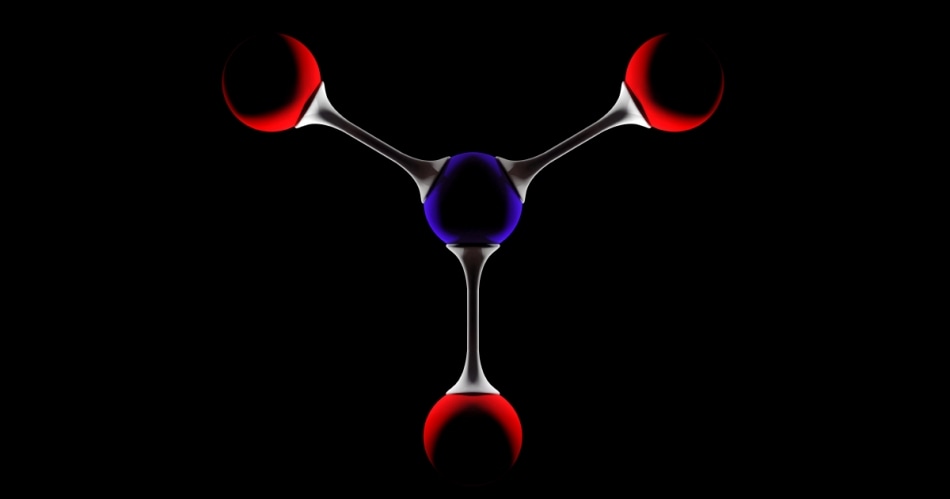
Compressing ammonia gas under high pressure forces the molecules into close We sometimes call a solid or a liquid a ‘condensed phase’. proximity. In a normal gas, the separation between each molecule is generally large – approximately 1000 molecular diameters is a good generalization. By contrast, the separation between the molecules in a condensed phase (solid or liquid) is more likely to be one to two molecular diameters, thereby explaining why the molar volume of a solid or liquid is so much smaller than the molar volume of a gas. As a direct consequence of the large intermolecular separations, ‘Intermolecular’ means ‘between molecules’.
we can safely say no interactions form between the molecules in ammonia gas. The molecules are simply too far apart. We saw in the previous chapter how the property known as pressure is a macroscopic manifestation of the microscopic collisions occurring between gas particles and, say, a solid object such as a container’s walls. But the gas particles can also strike each other on the same microscopic scale: we say the resultant interactions between molecules are intermolecular. Intermolecular interactions only operate over relatively short disA ‘formal bond’ involves the permanent involvement of electrons in covalent or ionic bonds; see p. 64. Interactions between molecules in a compressed gas are temporary. tances, so we assume that, under normal conditions, each molecule in a gas is wholly unaffected by all the others. By contrast,
when the gas is compressed and the particles come to within two or three molecular diameters of each other, they start to ‘notice’ each other. We say the outer-shell electrons on an atom are perturbed by the charges of the electrons on adjacent atoms, causing an interaction. We call these interactions bonds, even though they may be too weak to be formal bonds such as those permanently connecting the atoms or ions in a molecule. The intermolecular interactions between molecules of gas are generally attractive;
so, by way of response, we find that, once atoms are close enough to interact, they prefer to remain close – indeed, once a tentative interaction forms, the atoms ormolecules generally draw themselves closer, which itself makes Translational motion is movement through space, rather than a vibration about a mean point or a rotation about an axis. the interaction stronger. We see a simple analogy with everyday magnets: once two magnets are brought close enough to induce an interaction, we feel the attractive force dragging them closer still.
As soon as the particles of a gas attract, the inertia of the aggregate species increases, thereby slowing down all translational motion. And slower particles, such as these aggregates, are an easier target for further collisions than fast-moving gas atoms and molecules. The same principle explains why it is impossible to catch someone who is running very fast during a playground game of ‘tig’. Only when the runners tire and slow down can they be caught. In practice, as soon as an aggregate forms, we find that other gas particles soon adhere to it, causing eventual coalescence and the formation of a droplet of liquid. We say that nucleation occurs. With the same reasoning as that above, we can force the molecules of ammonia Formation of an aggregate facilitates further coalescence (eventually forming a condensed phase). We say ‘nucleation’ occurs. still closer together by applying a yet larger pressure, to form a denser state such as a solid.