In the previous example, we looked at the interactions induced when changing the external pressure, forcing the molecules into close proximity. We look here at the effects of changing the temperature. A bathroom mirror is usually colder than the temperature of the steam rising from No energy is exchanged during an ‘elastic’ collision, but energy is exchanged during an ‘inelastic’ collision. a hot bath. Each molecule of steam (gaseous water) has an enormous energy, which comes ultimately from the boiler that heats the water. The particles of steam would remain as liquid if they had less energy. In practice, particles evaporate from the bath to form energetic molecules of steam.
We see this energy as kinetic energy, so the particles move fast (see p. 30). The typical speeds at which gas particles move make it inevitable that steam molecules will collide with the mirror. We say such a collision is elastic if no energy transfers during the collision between the gas particle and the mirror; but if energy does transfer – and it usually does – we say the collision is inelastic. The energy transferred during an inelastic collision passes from the hot molecule of steam to the cooler mirror.
This energy flows in this direction because the steam initially possessed more energy per molecule than the mirror as a consequence of its higher temperature. It is merely a manifestation of the minus-oneth law of thermodynamics, as discussed in Chapter 1. But there are consequences to the collisions being inelastic: the molecules of steam have less energy following the collision because some of their energy has transferred We perceive this lower energy as a cooler temperature, meaning Energy is never lost or gained, only transferred or converted; see Chapter 3. that the water vapour in a steam-filled bathroom will cool down; conversely, the mirror (and walls) become warmer as they receive the energy that was previously possessed by the steam. These changes in the temperatures of gas and mirror occur in a complementary sense, so no energy is gained or lost. These changes in temperature represent a macroscopic proof that We generally assume that all particles in an ideal gas do not interact, meaning that the gas obeys the ideal-gas equation.
This assumption is sometimes poor. microscopic processes do occur. Indeed, it is difficult to envisage a transfer of energy between the gas particles with the cold mirror without these microscopic interactions. We spent quite a lot of time looking at the concept of an ideal gas in Chapter 1. The simplest definition of an ideal gas is that it obeys the ideal-gas equation (Equation (1.13)). Most gases can be considered as ideal most of the time. The most common cause of a gas disobeying the ideal-gas equation is the formation of interactions, and the results of intermolecular collisions.
Electronegativity and electropositivity
Liquid crystals are organic compounds that exhibit properties somewhere between those of a solid crystal and a liquid. Compounds I and II in Figure 2.1 both form liquid crystals at room temperature. We observe that liquid crystals can flow like any other viscous liquid, but they also possess some of the properties of crystalline solids, such as physical order, rather than random chaos. Unlike most other liquids, liquid crystals have some properties
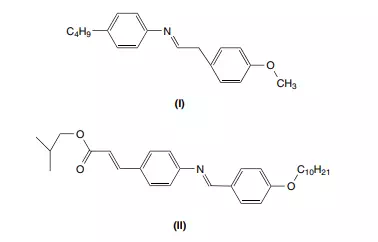
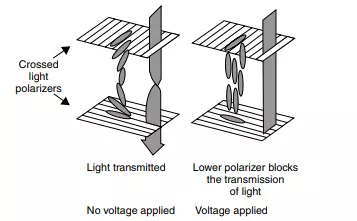
In a liquid-crystal display (LCD) device, the two electrodes A physicist would say the liquid crystal adopted a twisted nematic structure. are parallel and separated by a thin layer of liquid crystal (see Figure 2.2). The liquid crystals in this layer naturally adopt a helical structure. Light can be represented as a transverse electromagnetic wave made up of fluctuating electric and magnetic fields, moving in mutually perpendicular directions (see Chapter 9). Ordinary light is made up of waves that fluctuate at all possible angles, which normally cannot be separated. A polarizer is a material that allows only light with a specific angle of vibration to transmit. We place a light polarizer on one side of either transparent electrode in the LCD, each similar to one lens in a pair of polaroid sunglasses.
The helix of the liquid crystal twists the polarized light as it transmits through the LCD, guiding it from the upper polarizer and allowing it unhindered passage through the ‘sandwich’ and lower polarizer. The transmitting state of an LCD (at zero voltage) is thus ‘clear’. Applying a voltage to a pixel within the cell causes the mole- ‘Pixel’ is short for ‘picture element’. An LCD image comprises many thousands of pixels. cules to move, aligning themselves parallel with the electric field imparted by the electrodes. This realignment destroys the helical structure, precluding the unhindered transmission of light, and the display appears black.