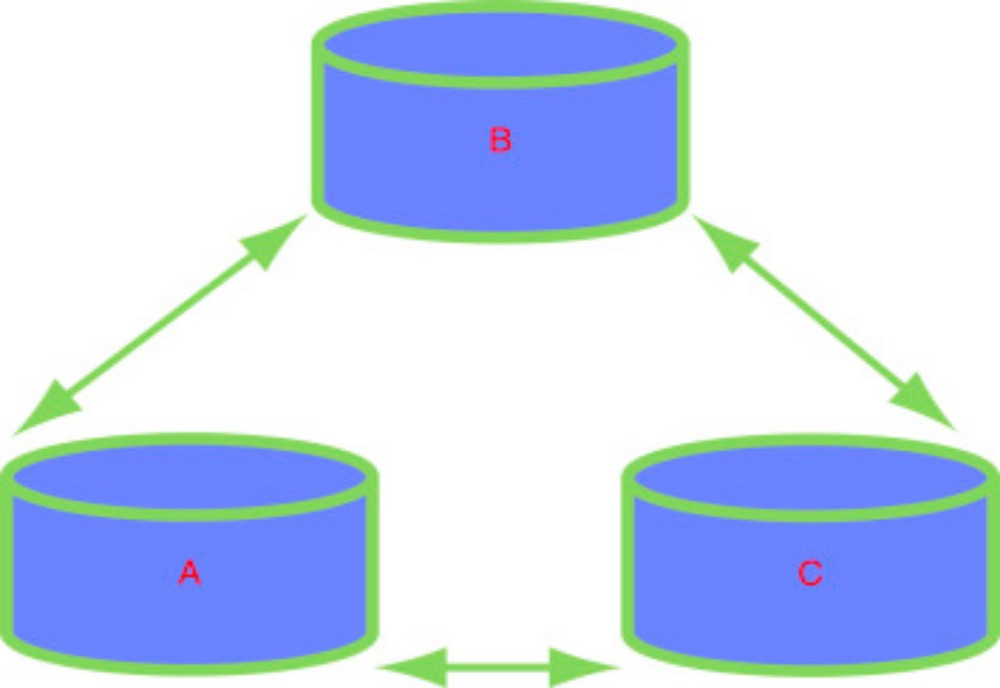
Feeling the temperature of a radiator is one of the simplest of A ‘law’ in physical chemistry relates to a wide range of situations. experiments. No one has ever sat in front of a hot radiator and felt colder. As a qualitative statement, we begin with the excellent generalization, ‘heat always travels from the hotter to the colder environment’. We call this observation a law because it is universal. Note how such a law is not concerned with magnitudes of change but simply relays information about a universal phenomenon: energy in the form of heat will travel from a hotter location or system to a place which is colder. Heat energy never travels in the opposite direction. We can also notice how, by saying ‘hotter’ and ‘colder’ rather than just ‘hot’ and ‘cold’, we can make the law wider in scope. The temperature of a radiator in a living room or lecture theatre is typically about 60 ◦ C, whereas a human body has an ideal temperature of about 37 ◦ C. The radiator is hotter than we are, so heat travels to us from the radiator. It is this heat emitted by the radiator which we absorb in order to feel warmer. Conversely, now consider placing your hands on a colder radiator having a temperature of 20 ◦ C (perhaps it is broken or has not been switched on). In this second example, although our hands still have the same temperature of 37 ◦ C, this time the heat energy travels to the radiator from our hands as soon as we touch it.
The direction of heat flow has been reversed in response to the reversal of the relative difference between the two temperatures. The direction in which the heat energy is transferred is one aspect of why the radiator feels cold. We see how the movement of energy not only has a magnitude but also a direction. Such statements concerning the direction of heat transfer are The ‘minus-oneth law of thermodynamics’ says, ‘heat always travels from hot to cold’. sometimes called the minus-oneth law of thermodynamics, which sounds rather daunting. In fact, the word ‘thermodynamics’ here may be taken apart piecemeal to translate it into everyday English. First the simple bit: ‘dynamic’ comes from the Greek word dunamikos, which means movement.
We obtain the conventional English word ‘dynamic’ from the same root; and a cyclist’s ‘dynamo’ generates electrical energy from the spinning of a bicycle wheel, i.e. from a moving object. Secondly, thermo is another commonly encountered Greek root, and means energy or temperature. We encounter the root thermo incorporated into such everyday words as ‘thermometer’, ‘thermal’ and ‘thermos flask’. A ‘thermodynamic’ property, therefore, relates to events or processes in which there are ‘changes in heat or energy’.