A fever is often the first visible sign of someone developing an The word ‘thermometer’ has two roots: meter denotes a device to measure something, and thermo means ‘energy’ or ‘temperature’. Thus, a ‘thermometer’ is a device for measuring energy as a function of temperature. illness. The body’s temperature rises – sometimes dramatically – above its preferred value of 37 ◦ C. As a good generalization, the temperature is hotter when the fever is worse, so it is wise to monitor the temperature of the sick person and thereby check the progress of the illness.
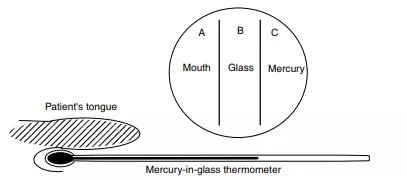
A thermometer is the ideal instrument for this purpose. When measuring a temperature with a thermometer, we place the mercury-containing end into the patient’s mouth or armpit and allow the reading to settle. The mercury is encased within a thinwalled glass tube, which itself is placed in contact with the patient. A ‘reading’ is possible because the mercury expands with increasing temperature: we take the length l of the mercury in the tube to be an accurate function of its temperature T . We read the patient’s temperature from the thermometer scale only when the length of the mercury has stopped changing.
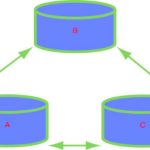
But how does the thermometer work in a thermodynamic sense, since at no time can the toxic mercury be allowed to touch the patient? Consider the flow of heat: heat energy first flows from the patient Bodies together at the same temperature are said to be in ‘thermal equilibrium’. to the glass, and thence flows through the glass into the mercury. Only when all three – mercury, glass and patient – are at the same temperature can the thermometer reading become steady.
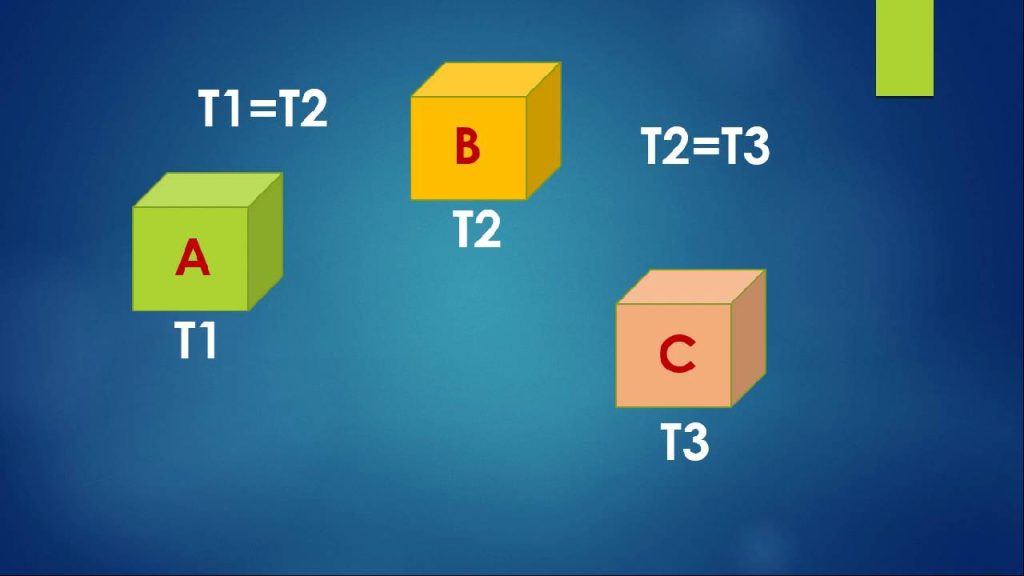
We say we have thermal equilibrium when these three have the same temperature; see Figure 1.3. Although in some respects a trivial example, a thermometer helps us see a profound truth: only when both (i) the mercury and the glass, and (ii) the glass and the patient are at thermal equilibrium can the patient and the mercury truly be said to be at the same temperature. By this means, we have measured the temperature of the patient by
utilizing a temperature-dependent property of the liquid metal inside the thermometer, yet at no time do we need to expose the patient to the toxic mercury. We begin to understand the power of thermodynamics when we The zeroth law of thermodynamics says: imagine three bodies, A, B and C. If A and B are in thermal equilibrium, and B and C are also in thermal equilibrium, then A and C will be in thermal equilibrium. realize how often this situation arises: in effect, we have made an indirect measurement – a frequent occurrence – so we need to formulate another law of thermodynamics, which we call the zeroth law. Imagine three bodies, A, B and C. If A and B are in thermal equilibrium, and B and C are in thermal equilibrium, then A and C are also in thermal equilibrium.
While sounding overly technical, we have in fact employed the zeroth law with the example of a thermometer. Let us rephrase the definition of the zeroth law and say, ‘If mercury is in thermal equilibrium with the glass of a thermometer, and the glass of a thermometer is in thermal equilibrium with a patient, then the mercury and the patient are also in thermal equilibrium’. A medic could not easily determine the temperature of a patient without this, the zeroth law. From now on we will assume the zeroth law is obeyed each time we use the phrase ‘thermal equilibrium’.