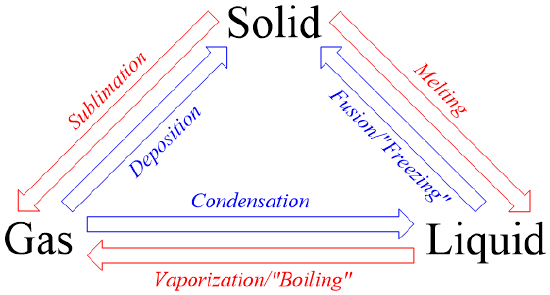
When we look around us, we see lots of changes happening in our day to day life.When we leave a glass of cold water unattended for some time, its surface gets fully covered with liquid droplets. When milk is kept on the gas flame, bubbles start to form, after attaining a particular temperature. A metal ring expands in diameter when it is heated over a flame, water turns solid when left in the freezer for some time and ice cream melts when left at room temperature. Have you ever wonder, why these changes take place? They are called as a Phase Change or a Phase Transition. Some of these changes like boiling, melting, condensation, etc are discussed below.
Boiling:
Boiling is the process by which, the liquid phase of a substance is converted into its gaseous phase. This process occurs with the increase in temperature to the boiling temperature or with the decrease in pressure to the atmospheric pressure.This process is used in cooking, purification of water, etc.

Condensation:
Condensation is the process by which, the physical state of a substance changes from its gas phase to liquid phase. It can also be defined as the transition of water vapour into water droplets, upon contacting a solid surface. This process is useful in separating a solute and solvent from its solution. The solution is heated, making the solvent to evaporate, which is then collected separately upon condensation.

Heating a Metal:
The structural, magnetic and electrical properties of metals, change upon heating. Metal expands when heated. Its length, volume and surface area increases. The process is termed as thermal expansion. The extent of thermal expansion differs with different metals. This process is useful when designing metallic structures, such as household pipes and fittings.

Freezing:
Freezing is the process by which the physical state of a substance changes from liquid to solid. This process of phase change takes place when the temperature is lowered so that liquid attains its freezing point. This process is used for the preservation of fruits and vegetables (frozen food) or animal specimen in the laboratories, as it slows the decay and growth of bacteria.
Melting:
Melting is a process that converts a substance from its solid phase to its liquid phase. This phase change takes place by the application of heat, which increases the temperature of the substance to its melting point. This process is used to remodel any substance to some other shape. For e.g. we melt metal blobs and recast them into different shapes of ring and block.


Comments are closed