The modern periodic table is based on the law that the properties of an element are a periodic function of their atomic number. These properties are related to the electronic configuration of the elements. We observe a common trend in properties as we move across a period from left to right or down the group. This trend in properties is known as periodic properties. The important periodic properties are atomic size, metallic character, non-metallic character, ionization potential, electron affinity, and electronegativity.
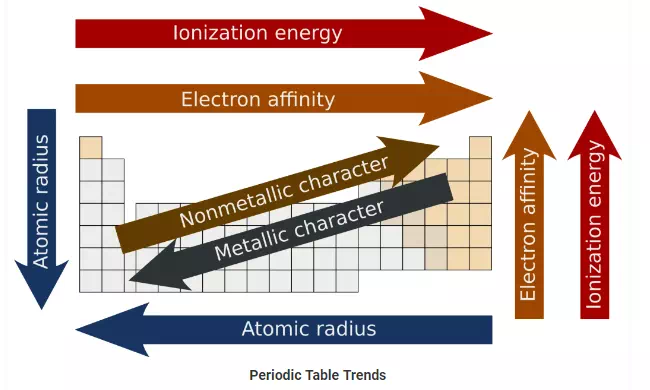
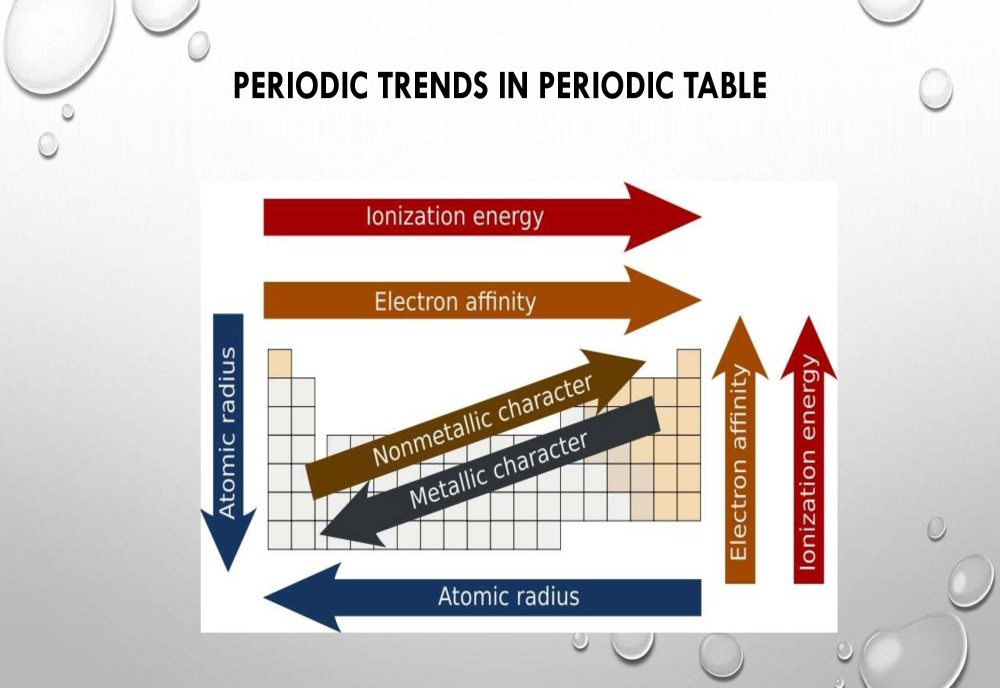
Periodic Table Trends:
The following trend in periodic properties of elements is observed:
Atomic size Trends:
The distance between the center of the nucleus and the outermost shell of an atom is known as the atomic radius. In a group the atomic size increases due to the addition of shells as we move from one period to another. Across a period the atomic size decreases as the number of shells remain the same while the nuclear charge increases. This leads to the pulling of electrons from the outermost shell towards the nucleus thereby decreasing the size.
Metallic character Trends:
The elements which lose electrons to form cations are known as metals. Metallic character increases as we move down the group because the atomic size increases which lead to easy loss of electrons. On the other hand, it decreases across a period as we move from left to right. This happens because there is an increase in nuclear charge which makes it difficult for an atom to lose electrons.
Non-metallic character Trends:
The elements which have a tendency to gain electrons are known as non-metals. The tendency to gain electrons increases on moving across a period due to an increase in the nuclear charge and decrease in the atomic size. Hence non-metallic character increases across a period. As we move down the group the non-metallic character decreases due to increase in the atomic size.
Ionization potential Trends:
Ionization potential is defined as the amount of energy required to remove an electron from the outermost shell of a gaseous atom and convert it into a positively charged gaseous ion.The periodic properties in terms of ionization potential increase because the atomic size decreases across a period due to increase in the nuclear charge. When we move down the group, ionization potential decreases due to the increase in atomic size.
Melting Point Trends:
The melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state. Which essentially implies breaking a few bonds. Thus, higher the stronger the bond between the atoms, higher will be the melting point.