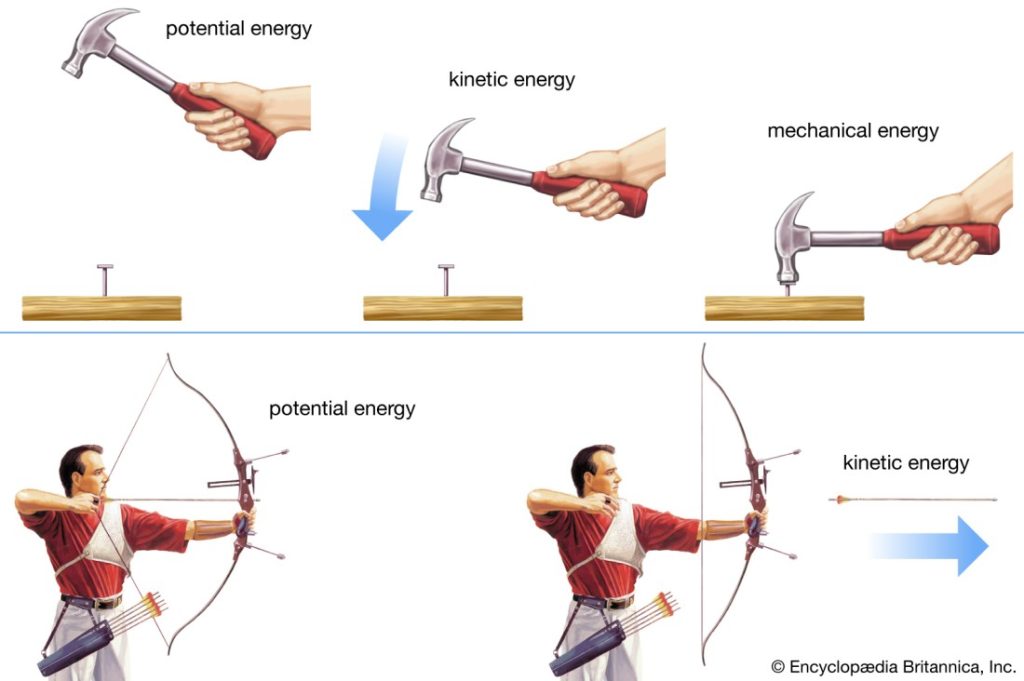
Potential energy of a system is due to the position of the system in a potential field. There are various forms of potential energy, but only gravitational potential energy will be considered in this course. The gravitational potential energy of an object of mass m at an elevation z in a gravitational field, relative to its gravitational potential energy at a reference elevation z0, is given by
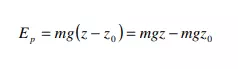
The quantity g is the gravitational acceleration that defines the strength of the gravitational field. Often, the earth’s surface is used as the reference and assigned z0 = 0, in which case mgz represents the gravitational potential energy of the object relative to its potential energy if it rested on the earth’s surface. Ep has units of energy, m of mass, g of length/time2 , and z of length.
Internal Energy
All the energy associated with a system that does not fall under the above definitions of kinetic or potential energy is internal energy. More specifically, internal energy is the energy due to all molecular, atomic, and subatomic motions and interactions. Usually, the complexity of these various contributions means that no simple analytical expression is available from which internal energy can be readily calculated. The internal energy will be represented by the symbol U. What types of events would bring about a change in a system’s internal energy? Is internal energy a state function?
Enthalpy
The enthalpy H of a system is defined by
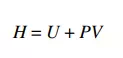
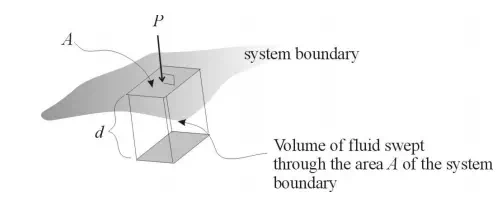
where P is the pressure and V is volume. Let’s think about the PV term. We know that PA, where A is the area subjected to a pressure P, is the force acting on that area. If a fluid inside a system is displaced through a distance d by the force PA, then the resultant work W done on the system can be calculated as the product of this force times the displacement. In other words, W = PAd. Now note that Ad = V, the volume swept out by the displacement. Thus, an alternate way to write the displacement work is W = PV. This type of work, where pressure results in the displacement of a fluid, will be referred to as flow work. If an amount of fluid of volume V is inserted into a system against a pressure P, the work required to accomplish this is PV. Enthalpy, therefore, can be viewed as the sum of the internal energy of this fluid volume added to the system plus the flow work performed on the system in order to insert the fluid. Enthalpy has units of energy (e.g. J, cal, BTU).