Abstract
Molecular oxygen is without doubt the greenest oxidant for redox reactions, yet aerobic oxidation is one of the most challenging to perform with good chemoselectivity, particularly on an industrial scale. This collaborative review (between teams of chemists and chemical engineers) describes the current scientific and operational hurdles that prevent the utilisation of aerobic oxidation reactions for the production of speciality chemicals and active pharmaceutical ingredients (APIs). The safety aspects of these reactions are discussed, followed by an overview of (continuous flow) reactors suitable for aerobic oxidation reactions that can be applied on scale. Some examples of how these reactions are currently performed in the industrial laboratory (in batch and in flow) are presented, with particular focus on the scale-up strategy. Last but not least, further challenges and future perspectives are presented in the concluding remarks.
Introduction
Molecular oxygen (O2) is unquestionably the most important constituent of our planet’s atmosphere. The photosynthesis of cyanobacteria began some 2.7 billion years ago, generating sufficient O2 in the atmosphere to support the evolution of more complex life forms. Today, many important biological functions are sustained by redox processes involving reactive oxygen species (ROS),1 including metabolism,2,3signalling4,5 and enzymatic functions.5
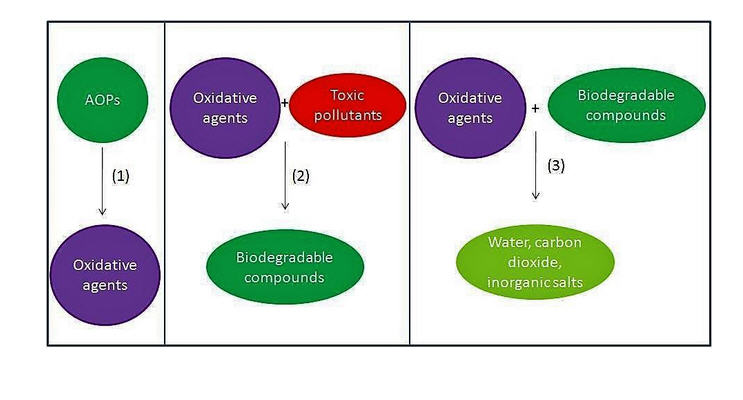
O2 can be considered as the ideal oxidant. It is readily abundant, has a low-molecular weight and, in most cases, generates only water as a benign by-product. Triplet, ground-state oxygen does not normally react with organic molecules under ambient conditions. However, the mixture may ignite under certain conditions to liberate CO2 and H2O, which is a highly exergonic process. Therefore, a catalyst is often required to control partial oxidation of a molecule selectively. In recent years, the demand for greener and more sustainable synthetic methods has generated much interest in the use of O2 as a reagent in synthesis.6 The subject of transition-metal catalysed oxidation of organic molecules has been comprehensively reviewed;7–9including, more recently, development of photocatalytic reactions (involving O2) driven by visible light,10 and the use of microreactors for liquid-phase oxidation chemistry.11 In general, there are two types of aerobic oxidation reactions, depending on the role played by O2 in the reaction (Scheme 1).
Scheme 1 Different types of aerobic oxidation reactions.
In Type I aerobic oxidation reactions, O2 is not incorporated into the product; its role is to regenerate the active catalyst and thus enable turnover to be achieved. By far, the dehydrogenation of alcohols to aldehydes and ketones constitutes the largest class of type I aerobic oxidation reactions. A wide variety of homogeneous and heterogeneous catalysts have been reported for this transformation, and the subject has been reviewed quite exhaustively. Aerobic oxidation also allows alcohols to be used as a latent source of carbonyl compounds which can be transformed into other functional groups (‘tandem oxidation processes’, TOP).
In Type II oxidation reactions, one or both of the oxygen atom(s) of O2 is/are incorporated into the product. The most common type II oxidation reactions are the oxygenation of C–H bonds, which proceed through free radical intermediates.12,13 The oxygenation of benzylic and allylic sp3 C–H bonds are particularly facile as they are highly susceptible to attack by free-radicals, which can be generated using cheap and abundant first-row transition metal salts (such as Fe, Co and Cu), N-hydroxyimides, light sensitive molecules, and even doped carbon materials.14 Enantioselective C–H oxygenation reactions can be achieved using enzymes,15 or their artificial mimics,16,17based on Fe.
Historically, organic chemists tend to eschew oxidation reactions in multistep organic synthesis as they are considered to be incompatible with the requirements of an ‘ideal synthesis’.18 Many oxidants, such as chromates and manganate, are often not chemoselective, and are difficult to implement on a large-scale. In an essay on redox economy in organic synthesis the authors advocated that the number of non-strategic redox steps should be avoided in multistep reactions.110 This view is very much echoed in an analysis of industrial reactions used for the preparation of 128 drug candidates between Pfizer, AstraZeneca and GlaxoSmithKline, where the need for more efficient oxidation chemistry has been identified as a key bottleneck in their processes: “…there are relatively few atom efficient, chemoselective and environmentally acceptable oxidation methods…oxidations are often designed out of syntheses. The discovery of new chemoselective oxidations, particularly if catalytic, would greatly increase flexibility in synthetic design”.19
Yet, paradoxically, oxidation reactions are commonly used processes for converting petrochemical feedstocks into useful chemical products. Similarly, there is also substantial interest in using aerobic oxidation reactions to convert biomass into platform chemicals.20 Currently, there are 109 industrial oxidation processes with a capacity of >1000 tonnes per annum that are listed in Ullmann’s Encyclopedia of Industrial Chemistry, including commodities, large intermediates and specialities (but excluding agrochemicals and pharmaceuticals).21 A survey of the processes listed (Fig. 1) shows that O2 is used as an oxidant in the majority of these processes (61.4%).
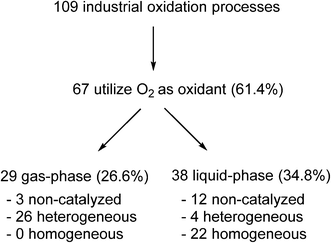
Fig. 1 Analysis of industrial oxidation processes with capacities of >1000 tonnes per annum.21
In general, bulk chemicals are simple molecules with few functional groups and low molecular weights/boiling points. This allows some reactions to be performed in the gas-phase at elevated temperatures and pressures (e.g. oxidation of ethylene to ethylene oxide), which are not compatible with the synthesis of more complex organic molecules required by the fine chemicals and pharmaceutical industries. Notably, the liquid-phase processes mostly utilise homogeneous catalysts: a large number of these are conducted in aqueous solutions, thus avoiding flammability issues associated with the use of organic solvents. An example is the production of synthetic vanillin. Currently, 95% of the world supply of this important flavouring agent is produced by the oxidation of petrochemical-based stock, mostly using the Riedel process (Scheme 2). Electrophilic substitution of guaiacol (1) with glyoxylic acid produces mandelic acid (2), which is oxidised to a vanilglyoxylic acid intermediate that decarboxylates spontaneously to vanillin (3). The conversion of 2 to 3 is carried out using a copper(ii) hydroxide-oxygen system in an aqueous alkaline medium at 80–130 °C.
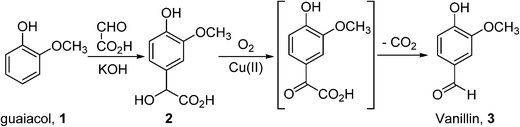
Scheme 2 The Riedel process for the production of artificial vanillin from guaiacol.
Indeed, very few homogeneous reactions listed in Fig. 1 are performed in organic solvents; a notable exception is the Amoco process for the production of terephthalic acid (which will be discussed in the following section). In fact, the lack of aerobic oxidation methodologies is more often due to safety concerns associated with aerated organic solvents. This may be demonstrated by menadione (4), a synthetic chemical compound widely used as an antihemorrhagic agent, as well as a nutritional supplement (Vitamin K3). This is currently prepared on an industrial scale by the oxidation of 2-methyl naphthalene (5) using chromate reagents in sulfuric acid (Scheme 3).22 However, the aerobic oxidation of 5 to 4 can also proceed uncatalyzed at 80 °C in toluene.23 Presumably, despite its obvious disadvantages (poor atom economy, toxic reagent, metal waste) the anaerobic oxidation is preferred as the exotherm can be controlled (by the slow addition of the reagent, for example), compared to performing an uncontrollable autooxidation process in a flammable solvent.
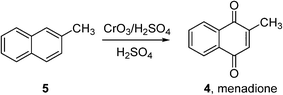
Scheme 3 Commercial synthesis of menadione (BASF).