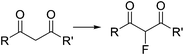
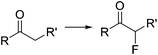
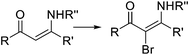
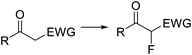Organoiodine compounds are uncommon in nature and not very frequently found in pharmaceuticals and agrochemicals (cf.Fig. 2). Until 2014, 184 natural iodinated compounds have been described.65 Yet, essential iodinated organic compounds such as thyroid hormones thyroxine T4 and triiodothyronine are produced by living organisms. Levothyroxine, a synthetic thyroid hormone used for the treatment of hypothyroidism, is one of the best selling drugs worldwide. Undoubtedly, iodination of organic compounds is most important for the generation of intermediates and useful building blocks for the preparation of more complex structures.
Iodination of organic materials using microreactor technology was first described by the group of Yoshida.66 Highly reactive I+ species were generated by electrolysis of I2and used for the electrophilic iodination of methoxybenzenes. The extremely fast reaction gave a 5 : 2 mixture of mono- and di-iodinated products in batch. The poor selectivity was ascribed to the inefficient mixing. Using a micromixer significantly improved the selectivities for the desired monoiodinated compounds (Scheme 11). Thus, a solution of the substrate in MeCN was mixed with the solution of I+ in MeCN generated electrochemically using a micromixer with 50 μm channel width cooled at 0 °C, before entering a microtube reactor to provide residence time. Using a flow rate of 3.0 mL for each reactant feed a selectivity of 95% for the monoiodinated compound was obtained. The effect of the flow rates on the reaction selectivity was very significant, decreasing when lower flow rates were applied due to the less efficient mixing (Scheme 11b).
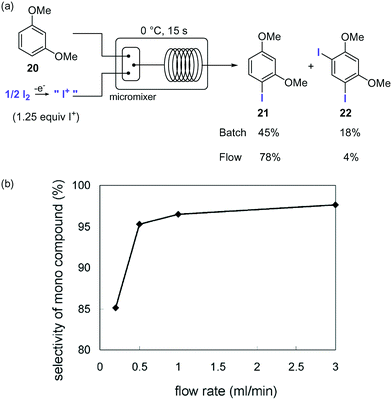
Scheme 11 Iodination of aromatics with electrochemically generated I+.
Elemental iodine (I2) has also been employed as a reagent for the direct iodination of aromatic compounds, in particular for the preparation of several 3-iodoindoles of type 24 (Scheme 12a).67 The simple flow setup consisted of two reagent feeds, containing the substrate and Et3N as base in DMF, and a solution of 1.1 equiv. I2 in DMF. The feeds were mixed using a T-mixer and reacted in a residence time unit made of 1 mm i.d. PTFE tubing. After a residence time of only 1 min at room temperature the reaction mixture was quenched with an aqueous solution of Na2S2O3 and NH4OH. Very good isolated yields were obtained for several 3-iodoindoles.
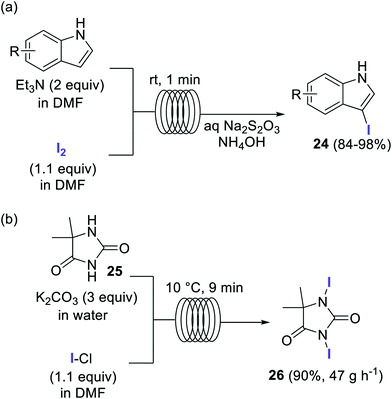
Scheme 12 Iodination of organic compounds using I2 and ICl as halogen source.
Iodine monochloride (ICl) is an excellent source of I+ frequently used for the iodination of aromatic compounds.68 Multi-100 gram scale preparation of the iodinating agent 26 was accomplished in a flow reactor using ICl as iodine source (Scheme 12b).69,70 A biphasic system comprising an aqueous solution of the substrate and K2CO3, and ICl in EtOAc was introduced into a tubular reactor at 10 °C. After a residence time of 9 min the slurry obtained from the reactor output was directly filtered using a semicontinuous filtration approach. The reactor was operated for 8 h with a yield of 90% for the pure product (375 g), which corresponds to a productivity of 47 g h−1.
Conclusions
Continuous flow chemistry offers unique opportunities to expand the array of synthetic methods available to the organic chemist. Chemistries and reagents classically “forbidden” under batch conditions, especially on a large scale due to safety reasons, become feasible using continuous flow and microreactor technology. Very fast and exothermic reactions and transformation that require highly reactive, toxic, or corrosive reagents are the prototype examples in which moving from classical batch reactor to flow is the best solution.71 As described in this review, halogenations are excellent examples of reactions that clearly benefit from the use of flow reactors. The most inexpensive, simple and atom economic halogenation procedures involve the use of highly reactive X2 and HX species. Organic chemists have traditionally searched for alternative, less reactive and less corrosive halogenating agents to carry out this type of transformations. Since the advent of flow chemistry numerous examples of highly reactive reagents such as F2 or Cl2 have been successfully utilized for the synthesis of organohalogens in a safe and controllable manner. Table 1 summarizes the reagents, target reactions, and typical experimental conditions for the continuous flow halogenations described herein. Further advantages of continuous flow halogenations arise for reaction involving gas/liquid biphasic mixtures, or photochemical halogenations that require light irradiation to proceed. Gas/liquid reactions are enhanced in continuous flow owing to the enhanced mass transfer and the fast mixing achieved. Thus, reactions involving the use of F2 or Cl2 gas gain in efficiency and selectivity as the reagents are dosed rapidly and homogeneously to the reaction mixture. Photochemical reactions are accelerated when glass chips or transparent tubing are used in flow, as very intense and uniform irradiation can be achieved. This has been demonstrated by several research groups for photochemical chlorinations with Cl2 and brominations with Br2 and NBS. Key to the advances accomplished in this field has been the implementation of microreactors built of corrosion resistant materials such as SiC or fluoropolymers, which allow the use of extremely aggressive halogenation reagents such as HF or F2. The application of these principles to other components of the flow reactors and equipment, such as pumping systems or back-pressure regulators is crucial to enable this technology to succeed on large scale preparations. Yet, a significant hazard often is the handling and storage of large quantities of toxic and corrosive halogenation reagents, even for their use in continuous flow. For that reason, the development of procedures for the continuous generation of halogenation reagents for their use in situ “on-demand” will be of interest in the future.
Table 1 Summary of reagents and conditions utilized for continuous flow halogenations
The development of the technology necessary for continuous flow synthesis during the past years, with a growing number of commercial equipment available, has enabled a very rapid growth of this area of research. A variety of rather creative chemistries have been explored in flow mode and, more importantly, many chemical reactions and reagents that had been “forbidden” or “forgotten” within the toolbox of organic synthesis due to safety reasons have been revisited with the application of continuous flow technology. Flow chemistry has demonstrated to be able to unconstrain chemical syntheses involving highly reactive reagents or very exothermic reactions, such as halogenations. Further innovations should be based in chemistries specifically developed to be performed in continuous flow.