A plant accumulates nutrients from the soil as it grows. Such accumulation depletes the amount of nutrient remaining in the soil; so, harvesting an arable crop, such as maize, barley or corn, removes nutrients from the field. A farmer needs to replenish the nutrients continually if the land is not to become ‘exhausted’ after a few seasons. In the context here, ‘nutrients’ principally comprise compounds.
The reaction of elemental nitrogen to form compounds that can be readily metabolized by a plant is termed ‘fixing’. All the principal means of fixing nitrogen involve bacteria. of nitrogen, most of which come from bacteria that employ naturally occurring catalysts (enzymes) which feed on elemental nitrogen – a process known as fixing. An example is the bacterium Rhizobium which lives on beans and peas. The bacteria convert atmospheric nitrogen into ammonia, which is subsequently available for important biological molecules such as amino acids, proteins, vitamins and nucleic acids.
Other than natural fixing, the principal sources of nutrients are the man-made fertilizers applied artificially by the farmer, the most common being inorganic ammonium nitrate (NH4NO3), which is unusually rich in nitrogen. But lightning is also an efficient fertilizer. The mixture of gases Notice the difference between the two words ‘princiPAL’ (meaning ‘best’, ‘top’ or ‘most important’) and ‘princiPLE’ (meaning ‘idea’, ‘thought’ or ‘concept’). we breathe comprises nitrogen (78 per cent), oxygen (21 per cent) and argon (1 per cent) as its principal components. The nitrogen atoms in the N2 molecule are bound together tightly via a triple bond, which is so strong that most reactions occurring during plant growth (photosynthesis) cannot cleave it: N2 is inert.
But the incredible energies unleashed by atmospheric lightning are able to overcome the N≡N bond. The actual mechanism by which the N≡N molecule cleaves is very complicated, and is not fully understood yet. It is nevertheless clear that much nitrogen is oxidized to form nitrous oxide, NO. This NO dissolves in the water that inevitably accompanies lightning and forms water-soluble nitrous acid HNO2, which further oxidizes during the storm to form nitric acid, HNO3. Nitric acid functions as a high-quality fertilizer. It has been estimated that a thunderstorm can yield many tonnes of fertilizer per acre of land.
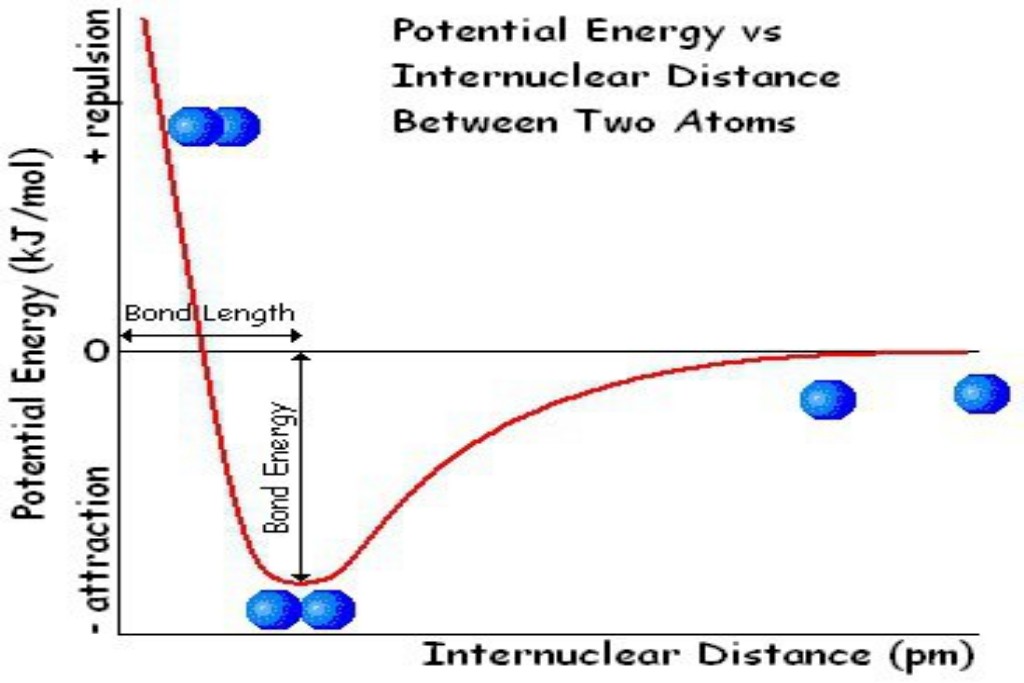
To summarize, the N≡N bond in the nitrogen molecule is very We require energy to cleave bonds: bond energies are discussed on p. 114. strong and cannot be cleaved unless a large amount of energy is available to overcome it. Whereas bacteria can fix nitrogen, the biological processes within crops, such as corn and maize, cannot provide sufficient energy. But the energy unleashed during a thunderstorm easily overcomes the N≡N bond energy, fixing the nitrogen without recourse to a catalyst.
Comments are closed