Eating ice cream soon causes the mouth to get cold, possibly to Ice cream melts as it warms in the mouth and surpasses its normal melting temperature; see Chapter 5. the extent of making it feel quite uncomfortable. The mouth of a normal, healthy adult has a temperature of about 37 ◦ C, and the ice cream has a maximum temperature of 0 ◦ C, although it is likely to be in the range −5 to −10 ◦ C if it recently came from the freezer. A large difference in temperature exists, so energy transfers from the mouth to the ice cream, causing it to melt.
The evidence for such a transfer of energy between the mouth and the ice cream is the change in temperature, itself a response to the minus-oneth law of thermodynamics (p. 7), which says heat travels from hot to cold. Furthermore, the zeroth law (p. 8) tells us energy will continue to transfer from the mouth (the hotter object) to the ice cream (the colder) until they are at the same temperature, i.e. when they are in thermal equilibrium.
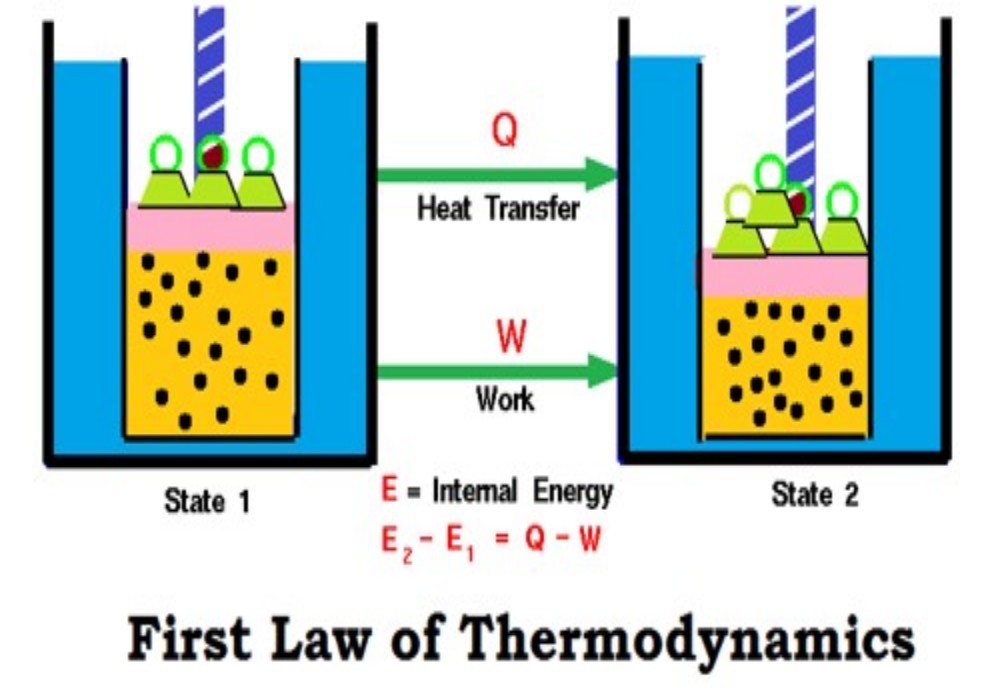
Internal energy U
Absolutely everything possesses energy. We cannot ‘see’ this energy directly, nor do we experience it except under certain conditions. It appears to be invisible because it is effectively ‘locked’ within a species. We call the energy possessed by the object the ‘internal energy’, and give it the symbol U. The internal energy U is defined as the total energy of a body’s We cannot know how much energy a body or system has ‘locked’ within it. Experimentally, we can only study changes in the internal energy, U. components.
Unfortunately, there is no way of telling how much energy is locked away. In consequence, the experimentalist can only look at changes in U. The energy is ‘locked up’ within a body or species in three prinThe energy E locked into the atomic nucleus is related to its mass m and the speed of light c, according to the Einstein equation, E = mc2. cipal ways (or ‘modes’). First, energy is locked within the atomic nuclei. The only way to release it is to split the nucleus, as happens in atomic weapons and nuclear power stations to yield nuclear energy.
The changes in energy caused by splitting nuclei are massive. We will briefly mention nuclear energy in Chapter 8, but the topic will not be discussed otherwise. It is too rare for most physical chemists to consider further. This second way in which energy is locked away is within chemical bonds. We call this form of energy the chemical energy, which is the subject of this chapter. Chemical energies are smaller than nuclear energies. And third, energy is possessed by virtue of the potential energy, and the translational, vibrational, rotational energy states of the atoms and bonds within the substance, be it atomic, molecular or ionic. The energy within each of these states is quantized, and will be discussed in greater detail in Chapter 9 within the subject of spectroscopy. These energies are normally much smaller than the energies of chemical bonds.
As thermodynamicists, we generally study the second of these Strictly, the bonds are held together with ‘outer-shell’ electrons. modes of energy change, following the breaking and formation of bonds (which are held together with electrons), although we occasionally consider potential energy. The magnitude of the chemical energy will change during a reaction, i.e. while altering the number and/or nature of the bonds in a chemical. We give the name calorimetry to the study of energy changes occurring during bond changes.
Chemists need to understand the physical chemistry underlying Placing the Greek letter (Delta) before the symbol for a parameter such as U indicates the change in U while passing from an initial to a final state. We define the change in a parameter X as X = X(final state) − X(initial state). these changes in chemical energy. We generally prefer to write in shorthand, so we don’t say ‘changes in internal energy’ nor the shorter phrase ‘changes in U’, but say instead ‘U’. But we need to be careful: the symbol does not just mean ‘change in’. We define it more precisely with Equation.

Comments are closed