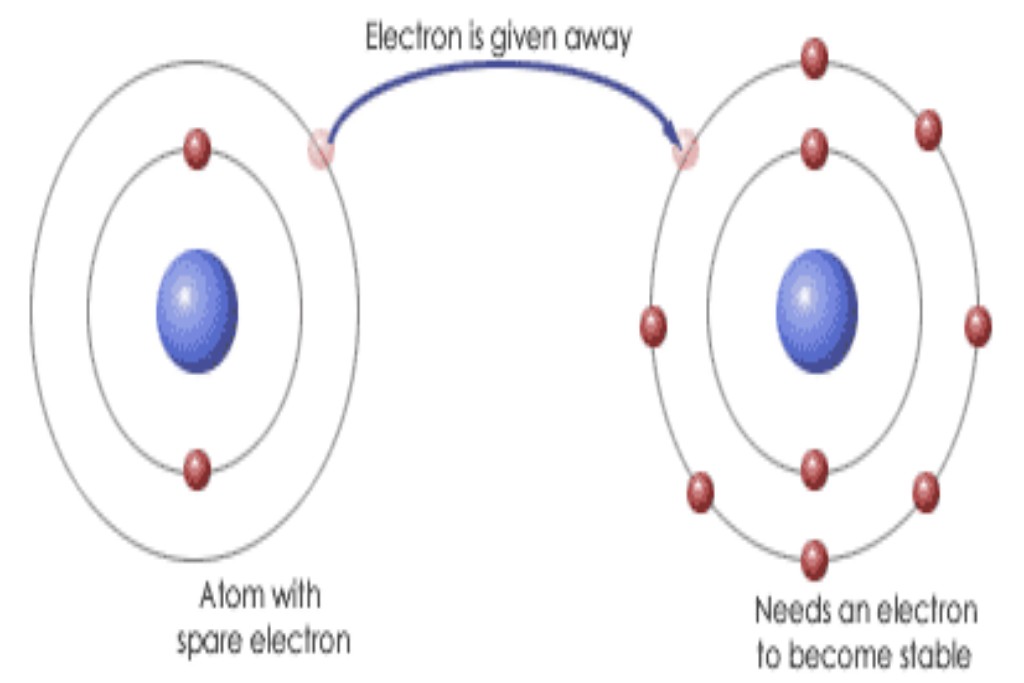
The water molecule always has a composition in which two hydrogen atoms combine with one oxygen. Why? The Lewis structure in Figure 2.11 represents water, H2O. Oxygen is element number eight in the periodic table, and each oxygen atom possesses six electrons in its outer shell. Being a member of the second row of the periodic table, each oxygen atom seeks to have an outer shell of eight electrons – we call this trend the ‘octet rule’. Each oxygen atom, therefore, has a deficiency of two electrons. As we saw immediately above, an atom of hydrogen has a single electron and, being a row 1 element, requires just one more to complete its outer shell.
The Lewis structure in Figure 2.12 shows the simplest way in The concept of the full outer shell is crucial if we wish to understand covalent bonds. which nature satisfies the valence requirements of each element: each hydrogen shares its single electron with the oxygen, meaning that the oxygen atom now has eight electrons (six of its own – the crosses in the figure) and two from the hydrogen atoms. Looking now at each hydrogen atom (the two are identical), we see how each now has two electrons: its own original electron (the dot in the diagram) together with one extra electron each from the oxygen (depicted as crosses). We have not increased the number of electrons at all. All we have done is shared them between the two elements, thereby enabling each atom to have a full outer shell. This approach is known as the electron-pair theory.
Valence bond theory
The valence bond theory was developed by Linus Pauling (1901–1992) and others in the 1930s to amalgamate the existing electron-pair bonding theory of G. N. Lewis and new data concerning molecular geometry. Pauling wanted a single, unifying theory. He produced a conceptual framework to explain molecular bonding, but in practice it could not explain the shapes of many molecules.
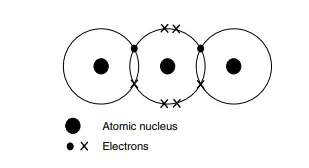
Nevertheless, even today, we often discuss the bonding of organic compounds in terms of Lewis structures and valence bond theory.