Clear petroleum gel is a common product, comprising a mixture of simple hydrocarbons, principally n-octadecane (III). It is not quite a solid at room temperature; neither is it really a liquid, because it is very viscous. We call it a gel. Its principal applications are to lubricate (in a car) or to act as a water-impermeable barrier (e.g. between a baby and its nappy, or on chapped hands).
We saw on p. 52 how methane is a gas unless condensed by compression at high pressure or frozen to low temperatures. But octadecane is neither a solid nor a gas. Why? There are several, separate types of interaction in III: both covaMolecules made of only one element are called ‘homonuclear’, since homo is Greek for ‘same’. Examples of homonuclear molecules are H2, N2,S8 and fullerene C60. lent bonds and dipoles. Induced dipoles involve a partial charge, which we called δ+ or δ−, but, by contrast, covalent bonds involve whole numbers of electrons. A normal covalent bond, such as that between a hydrogen atom and one of the carbon atoms in the backbone of III, requires two electrons. A ‘double bond’ consists simply of two covalent bonds, so four electrons are shared. Six electrons are incorporated in each of the rare instances of a covalent ‘triple bond’. A few quadruple bonds occur in organometallic chemistry, but we will ignore them here.
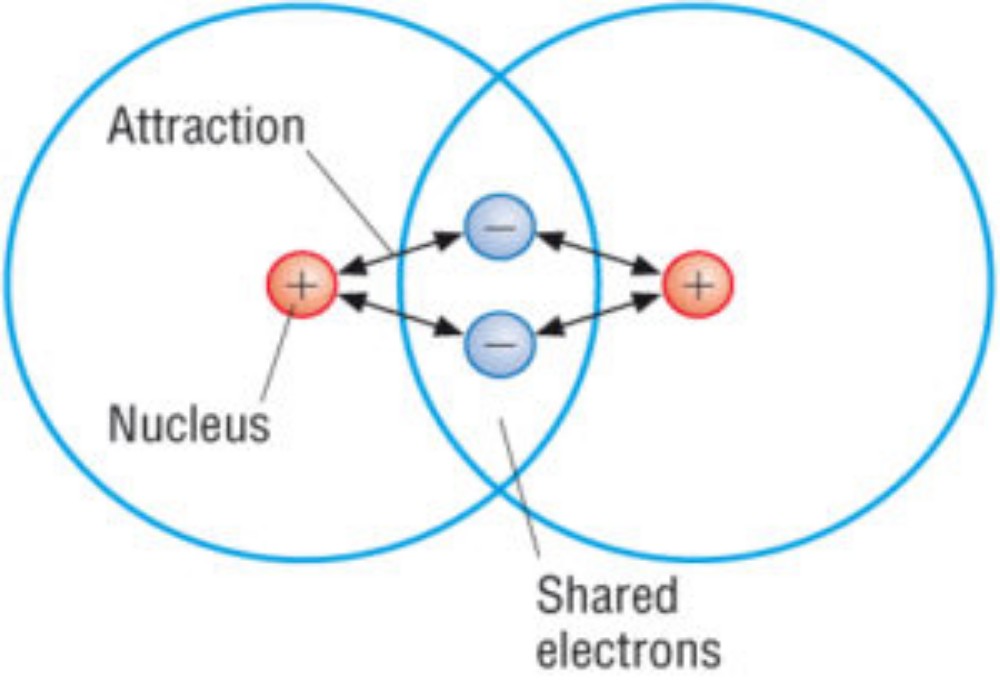
Most covalent bonds are relatively non-polar. Some are comEven a covalent bond can possess a permanent induced dipole. Covalent compounds tend to be gases or liquids. Even when solid, they tend to be soft. But many covalent compounds are only solid at lower temperatures and/or higher pressures, i.e. by maximizing the incidence of induced dipoles. pletely non-polar: the diatomic hydrogen molecule is held together with two electrons located equidistantly from the two hydrogen nuclei. Each of the two atoms has an equal ‘claim’ on the electrons, with the consequence that there is no partial charge on the atoms: each is wholly neutral. Only homonuclear molecules such as H2, F2, O2 or N2 are wholly non-polar, implying that the majority of covalent bonds do possess a slight polarity, arising from an unequal sharing of the electrons bound up within the bond. We see the possibility of a substance having several types of bond.
Consider water for example. Formal covalent bonds hold together the hydrogen and oxygen atoms, but the individual water molecules cohere by means of hydrogen bonds. Conversely, paraffin wax (n-C15H32) is a solid. Each carbon is bonded covalently.
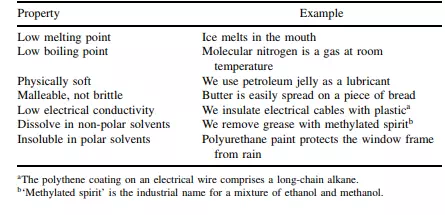
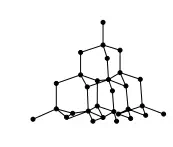
to one or two others to form a linear chain; the hydrogen atoms are bound to this backbone, again with covalent bonds. But the wax is a solid because dispersion forces ‘glue’ together the molecules. Table 2.6 lists some of the common properties of covalent compounds. Finally, macromolecular covalent solids are unusual in comprising atoms held together in a gigantic three-dimensional array of bonds. Diamond and silica are the simplest examples; see Figure 2.13. Giant macroscopic structures are always solid.