In comic books of the 1950s, one of the favourite phrases of super-heroes such as Superman was ‘greased lightning!’ The idea is one of extreme speed. The lightning we see, greased or otherwise, is a form of light and travels very, very fast. For example, it travels through a vacuum at 3 × 108 m s−1, which we denote with the symbol c. But while the speed c is constant, the actual speed of light may not be: in fact, it alters very slightly depending on the medium through which it travels. We see how a definition of time involving the speed of light is inherently risky, explaining why we now choose to define time in terms of the duration (or fractions and multiples thereof) between static events. And by ‘static’ we mean unchanging.
SI ‘base units’
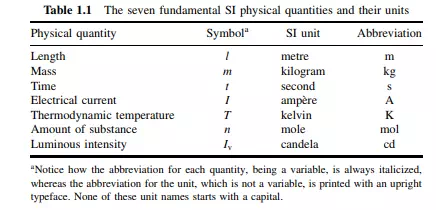
Time is one of the so-called ‘base units’ within the SI system, and so is length. There are seven base SI units: length, time, mass, temperature, current, luminous intensity and amount of material. Whereas volume can be expressed in terms of a length (for example, a cube has a volume l 3 and side of area l 2), we cannot define length in terms of something simpler. Similarly, whereas a velocity is a length per unit time, we cannot express time in terms of something simpler. In fact, just as compounds are made up of elements, so all scientific units are made up from seven base units: length, time, mass, temperature, current, amount of material and luminous intensity. Table 1.1 summarizes the seven base (or ‘fundamental’) SI physical quantities and their units. The last unit, luminous intensity, will not require our attention any further. The SI unit of ‘time’ t is the second. The second was originally The SI unit of ‘time’ t is the second (s). defined as 1/86 400th part of a mean solar day.
In a similar way, the Syst`eme Internationale has ‘defined’ other The SI unit of ‘temperature’ T is the kelvin (K). common physicochemical variables. The SI unit of ‘temperature’ T is the kelvin. We define the kelvin as 1/273.16th part of the thermodynamic temperature difference between absolute zero (see Section 1.4) and the triple point of water, i.e. the temperature at which liquid water is at equilibrium with solid water (ice) and gaseous water (steam) provided that the pressure is 610 Pa. The SI unit of ‘current’ I is the ampere (A). An amp ` ere was ` The SI unit of ‘current’ I is the amp`ere (A).
first defined as the current flowing when a charge of 1 C (coulomb) passed per second through a perfect (i.e. resistance-free) conductor. The SI definition is more rigorous: ‘the ampere is that constant ` current which, if maintained in two parallel conductors (each of negligible resistance) and placed in vacuo 1 m apart, produces a force between of exactly 2 × 10−7 N per metre of length’. We will not employ this latter definition. The SI unit of the ‘amount of substance’ n is the mole. CuriThe SI unit of ‘amount of substance’ n is the mole (mol). ously, the SI General Conference on Weights and Measures only decided in 1971 to incorporate the mole into its basic set of fundamental parameters, thereby filling an embarrassing loophole. The mole is the amount of substance in a system that contains as many elementary entities as does 0.012 kg (12 g) of carbon-12. The amount of substance must be stated in terms of the elementary entities chosen, be they photons, electrons, protons, atoms, ions or molecules.