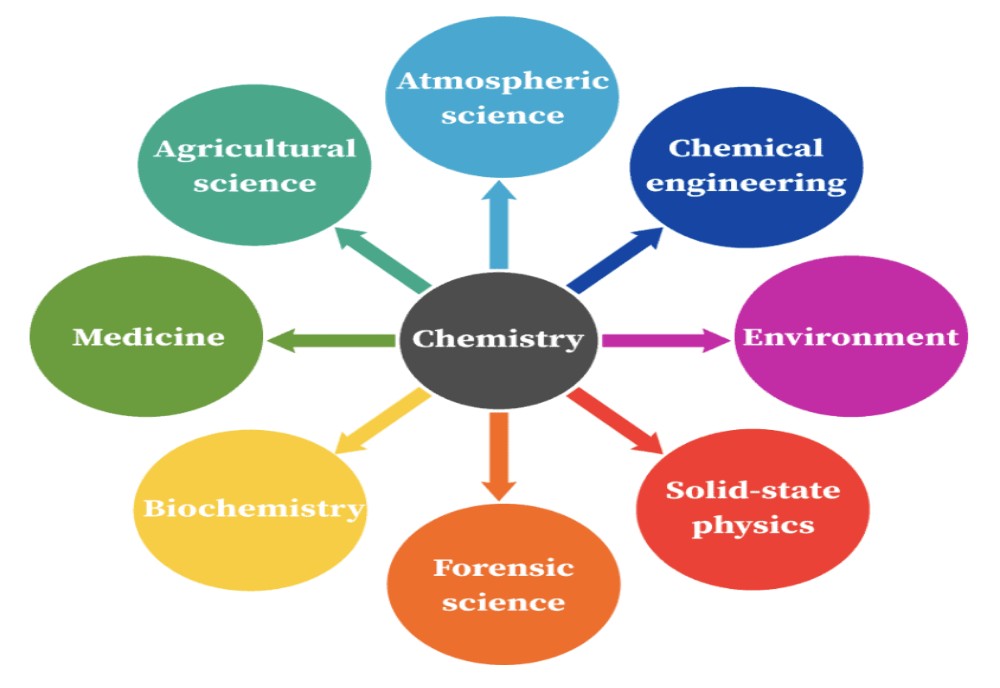
The days are long past when one person could hope to have a detailed knowledge of all areas of chemistry. Those pursuing their interests into specific areas of chemistry communicate with others who share the same interests. Over time a group of chemists with specialized research interests become the founding members of an area of specialization. The areas of specialization that emerged early in the history of chemistry, such as organic, inorganic, physical, analytical, and industrial chemistry, along with biochemistry, remain of greatest general interest. There has been, however, much growth in the areas of polymer, environmental, and medicinal chemistry during the 20th century. Moreover, new specialities continue to appear, as, for example, pesticide, forensic, and computer chemistry.
Analytical chemistry
Most of the materials that occur on Earth, such as wood, coal, minerals, or air, are mixtures of many different and distinct chemical substances. Each pure chemical substance (e.g., oxygen, iron, or water) has a characteristic set of properties that gives it its chemical identity. Iron, for example, is a common silver-white metal that melts at 1,535° C, is very malleable, and readily combines with oxygen to form the common substances hematite and magnetite. The detection of iron in a mixture of metals, or in a compound such as magnetite, is a branch of analytical chemistry called qualitative analysis. Measurement of the actual amount of a certain substance in a compound or mixture is termed quantitative analysis. Quantitative analytic measurement has determined, for instance, that iron makes up 72.3 percent, by mass, of magnetite, the mineral commonly seen as black sand along beaches and stream banks. Over the years, chemists have discovered chemical reactions that indicate the presence of such elemental substances by the production of easily visible and identifiable products. Iron can be detected by chemical means if it is present in a sample to an amount of 1 part per million or greater. Some very simple qualitative tests reveal the presence of specific chemical elements in even smaller amounts. The yellow colour imparted to a flame by sodium is visible if the sample being ignited has as little as one-billionth of a gram of sodium. Such analytic tests have allowed chemists to identify the types and amounts of impurities in various substances and to determine the properties of very pure materials. Substances used in common laboratory experiments generally have impurity levels of less than 0.1 percent. For special applications, one can purchase chemicals that have impurities totaling less than 0.001 percent. The identification of pure substances and the analysis of chemical mixtures enable all other chemical disciplines to flourish.
The importance of analytical chemistry has never been greater than it is today. The demand in modern societies for a variety of safe foods, affordable consumer goods, abundant energy, and labour-saving technologies places a great burden on the environment. All chemical manufacturing produces waste products in addition to the desired substances, and waste disposal has not always been carried out carefully. Disruption of the environment has occurred since the dawn of civilization, and pollution problems have increased with the growth of global population. The techniques of analytical chemistry are relied on heavily to maintain a benign environment. The undesirable substances in water, air, soil, and food must be identified, their point of origin fixed, and safe, economical methods for their removal or neutralization developed. Once the amount of a pollutant deemed to be hazardous has been assessed, it becomes important to detect harmful substances at concentrations well below the danger level. Analytical chemists seek to develop increasingly accurate and sensitive techniques and instruments.
Sophisticated analytic instruments, often coupled with computers, have improved the accuracy with which chemists can identify substances and have lowered detection limits. An analytic technique in general use is gas chromatography, which separates the different components of a gaseous mixture by passing the mixture through a long, narrow column of absorbent but porous material. The different gases interact differently with this absorbent material and pass through the column at different rates. As the separate gases flow out of the column, they can be passed into another analytic instrument called a mass spectrometer, which separates substances according to the mass of their constituent ions. A combined gas chromatograph–mass spectrometer can rapidly identify the individual components of a chemical mixture whose concentrations may be no greater than a few parts per billion. Similar or even greater sensitivities can be obtained under favourable conditions using techniques such as atomic absorption, polarography, and neutron activation. The rate of instrumental innovation is such that analytic instruments often become obsolete within 10 years of their introduction. Newer instruments are more accurate and faster and are employed widely in the areas of environmental and medicinal chemistry.

Comments are closed