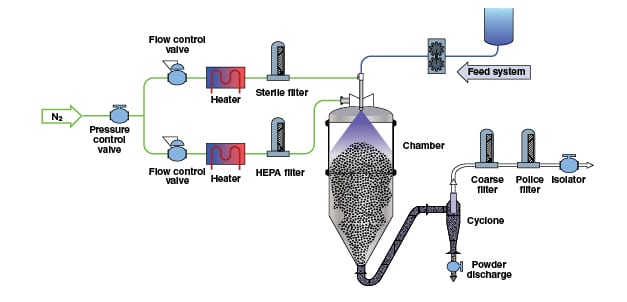
Adjustment and control of moisture levels in solid materials through drying is a critical process in the manufacture of many types of chemical products. As a unit operation, drying solid materials is one of the most common and important in the chemical process industries (CPI), since it is used in practically every plant and facility that manufactures or handles solid materials, in the form of powders and granules.
The effectiveness of drying processes can have a large impact on product quality and process efficiency in the CPI. For example, in the pharmaceutical industry, where drying normally occurs as a batch process, drying is a key manufacturing step. The drying process can impact subsequent manufacturing steps, including tableting or encapsulation and can influence critical quality attributes of the final dosage form.
Apart from the obvious requirement of drying solids for a subsequent operation, drying may also be carried out to improve handling characteristics, as in bulk powder filling and other operations involving powder flow; and to stabilize moisture-sensitive materials, such as pharmaceuticals. This article provides basic information on the sometimes complicated heat- and mass-transfer processes that are important in drying, and discusses several technologies used to accomplish the task.
MECHANISM OF DRYING
Drying may be defined as the vaporization and removal of water or other liquids from a solution, suspension, or other solid-liquid mixture to form a dry solid. It is a complicated process that involves simultaneous heat and mass transfer, accompanied by physicochemical transformations. Drying occurs as a result of the vaporization of liquid by supplying heat to wet feedstock, granules, filter cakes and so on. Based on the mechanism of heat transfer that is employed, drying is categorized into direct (convection), indirect or contact (conduction), radiant (radiation) and dielectric or microwave (radio frequency) drying.
Heat transfer and mass transfer are critical aspects in drying processes. Heat is transferred to the product to evaporate liquid, and mass is transferred as a vapor into the surrounding gas. The drying rate is determined by the set of factors that affect heat and mass transfer. Solids drying is generally understood to follow two distinct drying zones, known as the constant-rate period and the falling-rate period. The two zones are demarcated by a break point called the critical moisture content.
In a typical graph of moisture content versus drying rate and moisture content versus time (Figure 1), section AB represents the constant-rate period. In that zone, moisture is considered to be evaporating from a saturated surface at a rate governed by diffusion from the surface through the stationary air film that is in contact with it. This period depends on the air temperature, humidity and speed of moisture to the surface, which in turn determine the temperature of the saturated surface. During the constant rate period, liquid must be transported to the surface at a rate sufficient to maintain saturation.
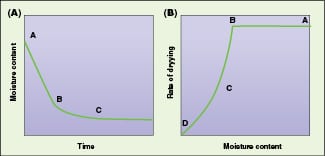
Figure 1. Segment AB of the graph represents the constant-rate drying period, while segment BC is the falling-rate period
At the end of the constant rate period, (point B, Figure 1), a break in the drying curve occurs. This point is called the critical moisture content, and a linear fall in the drying rate occurs with further drying. This section, segment BC, is called the first falling-rate period. As drying proceeds, moisture reaches the surface at a decreasing rate and the mechanism that controls its transfer will influence the rate of drying. Since the surface is no longer saturated, it will tend to rise above the wet bulb temperature. This section, represented by segment CD in Figure 1 is called the second falling-rate period, and is controlled by vapor diffusion. Movement of liquid may occur by diffusion under the concentration gradient created by the depletion of water at the surface. The gradient can be caused by evaporation, or as a result of capillary forces, or through a cycle of vaporization and condensation, or by osmotic effects.
The capacity of the air (gas) stream to absorb and carry away moisture determines the drying rate and establishes the duration of the drying cycle. The two elements essential to this process are inlet air temperature and air flowrate. The higher the temperature of the drying air, the greater its vapor holding capacity. Since the temperature of the wet granules in a hot gas depends on the rate of evaporation, the key to analyzing the drying process is psychrometry, defined as the study of the relationships between the material and energy balances of water vapor and air mixture.
DRYING ENDPOINT
There are a number of approaches to determine the end of the drying process. The most common one is to construct a drying curve by taking samples during different stages of drying cycle against the drying time and establish a drying curve. When the drying is complete, the product temperature will start to increase, indicating the completion of drying at a specific, desired product-moisture content. Karl Fischer titration and loss on drying (LOD) moisture analyzers are also routinely used in batch processes. The water vapor sorption isotherms are measured using a gravimetric moisture-sorption apparatus with vacuum-drying capability.
For measuring moisture content in grain, wood, food, textiles, pulp, paper, chemicals, mortar, soil, coffee, jute, tobacco, rice and concrete, electrical-resistance-type meters are used. This type of instrument operates on the principle of electrical resistance, which varies minutely in accordance with the moisture content of the item measured. Dielectric moisture meters are also used. They rely on surface contact with a flat plate electrode that does not penetrate the product.
For measuring moisture content in paper rolls or stacks of paper, advanced methods include the use of the radio frequency (RF) capacitance method. This type of instrument measures the loss, or change, in RF dielectric constant, which is affected by the presence or absence of moisture.
TYPES OF DRYERS
Adiabatic dryers are the type where the solids are dried by direct contact with gases, usually forced air. With these dryers, moisture is on the surface of the solid. Non-adiabatic dryers involve situations where a dryer does not use heated air or other gases to provide the energy required for the drying process
Dryer classification can also be based on the mechanisms of heat transfer as follows:
• Direct (convection)
• Indirect or contact (conduction)
• Radiant (radiation)
• Dielectric or microwave (radio frequency) drying
Direct, or adiabatic, units use the sensible heat of the fluid that contacts the solid to provide the heat of vaporization of the liquid.
With adiabatic dryers, solid materials can be exposed to the heated gases via various methods, including the following:
• Gases can be blown across the surface (cross circulation)
• Gases can be blown through a bed of solids (through-circulation); used when solids are stationary, such as wood, corn and others
• Solids can be dropped slowly through a slow-moving gas stream, as in a rotary dryer
• Gases can be blown through a bed of solids that fluidize the particles. In this case, the solids are moving, as in a fluidized-bed dryer
• Solids can enter a high-velocity hot gas stream and can be conveyed pneumatically to a collector (flash dryer)
Non-adiabatic dryers (contact dryers) involve an indirect method of removal of a liquid phase from the solid material through the application of heat, such that the heat-transfer medium is separated from the product to be dried by a metal wall. Heat transfer to the product is predominantly by conduction through the metal wall and the impeller. Therefore, these units are also called conductive dryers.
Although more than 85% of the industrial dryers are of the convective type, contact dryers offer higher thermal efficiency and have economic and environmental advantages over convective dryers. Table 1 compares direct and indirect dryers, while Table 2 shows the classification of dryers based on various criteria.
| Table 1. Comparison of Direct and Indirect dryers | ||
| Property | Direct/adiabatic dryer (convective type) | Indirect/non-adiabatic contact dryer (conductive type) |
| Carrier gas | Uses sensible heat of gas that contacts the solid to provide the heat of vaporization of the liquid | Little or no carrier gas is required to remove the vapors released from the solids |
| Heat transfer | Heat transfer medium is in direct contact with the surface of the material to be dried | Heat needed to vaporize the solvent is transferred through a wall |
| Risk of cross contamination | Persists | Avoided, as the heat transfer medium does not contact the product |
| Solvent recovery | Difficult as there is a large volume of gas to be cooled to recover the solvent | Easier because of limited amount of non-condensable gas encountered |
| Operation under vacuum | Not possible | Allows operation under vacuum, ideal for heat sensitive materials |
| Dusting | High | Minimized because of small volume of vapors involved |
| Explosion hazard | Higher rate | Easier to control as vapors can be easily condensed |
| Handling of toxic materials | Not suitable | Suitable because of low gas flow |
| Energy efficiency | Significant energy lost through exhaust gas | Higher energy efficiency as the energy lost through the exhaust gas is greatly reduced |
| Evaporation and production rates | Higher than contact dryers | Drying rates are limited by heat transfer area, lower production rates |
| Cost | High | Higher initial cost; difficult to design, fabricate and maintain |
| Table 2. Classification of Dryers | |
| Criterion | Types |
| Mode of operation | Batch Continuous* |
| Heat input type | Convection*, conduction, radiation, electromagnetic fields, combination of heat transfer modes Intermittent or continuous* Adiabatic or non-adiabatic |
| State of material in dryer | Stationary Moving agitated, dispersed |
| Operating pressure | Vacuum* Atmospheric |
| Drying medium (convection) | Air* Superheated steam Fluegases |
| Drying temperature | Below boling temperature* Above boiling temperature Below freezing point |
| Relative motion between drying medium and solids | Co-current Countercurrent Mixed flow |
| Number of stages | Single* Multistage |
| Residence time | Short (60 min) |
| * Most common in practice |