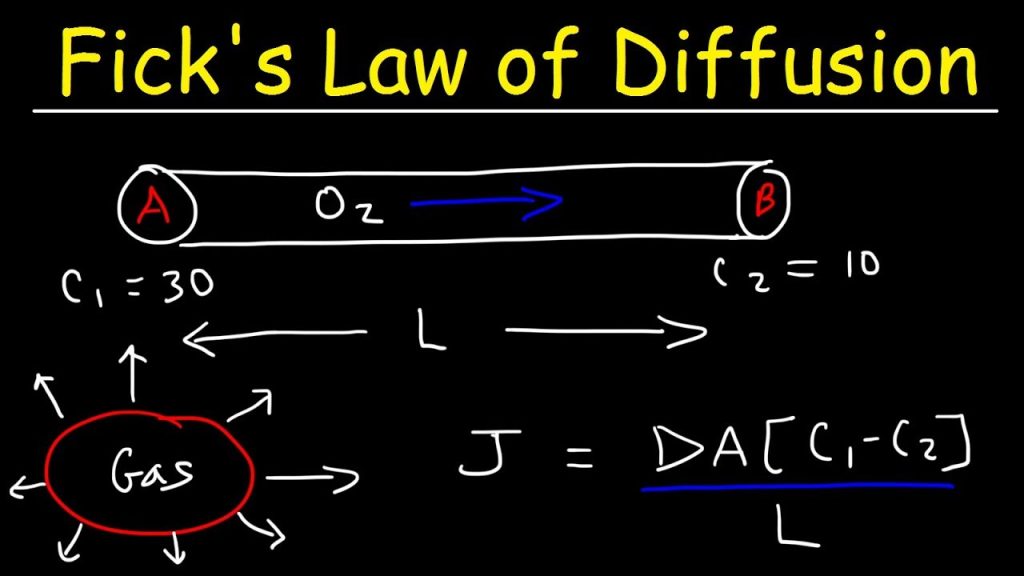
Adolf Fick (1955) first described the molecular diffusion in an isothermal, isobaric binary system of components A and B[1-3]. According to his idea of molecular diffusion, the molar flux of a species relative to an observer moving with molar average velocity is proportional to the concentration gradient in a certain direction.

Where, JA is the molar flux of component A in the Z direction. CA is the concentration of A and Z is the distance of diffusion. The proportionality constant, DAB is the diffusion coefficient of the molecule A in B. This is valid only at steady state condition of diffusion. The Equation (2.2) is called Fick’s first law of diffusion. If the concentration gradient is expressed as the gradient of mole fraction and in three dimensional cases, the molar flux can be expressed as

Substituting the Equation (2.2) for JA into Equation (1.21) in module 1, the molar flux with negligible bulk movement of component A of the binary gas mixture can be represented as