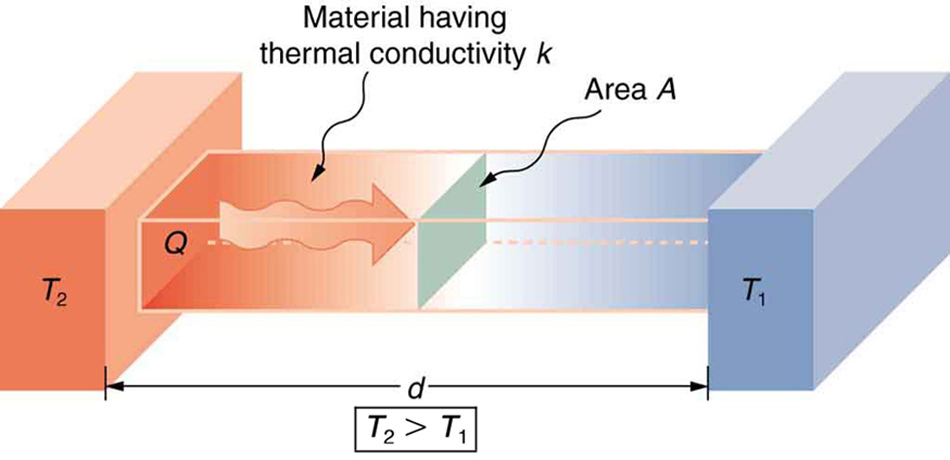
As noted previously, thermal conductivity is a thermodynamic property of a material. From the State Postulate given in thermodynamics, it may be recalled that thermodynamic properties of pure substances are functions of two independent thermodynamic intensive properties, say temperature and pressure. Thermal conductivity of real gases is largely independent of pressure and may be considered a function of temperature alone. For solids and liquids, properties are largely independent of pressure and depend on temperature alone.
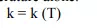
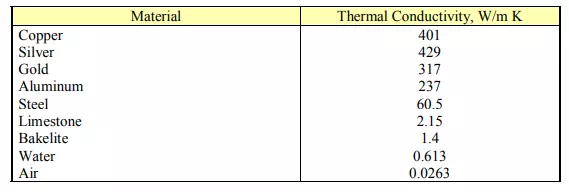
Let us try to gain an insight into the basic concept of thermal conductivity for various materials. The fundamental concept comes from the molecular or atomic scale activities. Molecules/atoms of various materials gain energy through different mechanisms. Gases, in which molecules are free to move with a mean free path sufficiently large compared to their diameters, possess energy in the form of kinetic energy of the molecules. Energy is gained or lost through collisions/interactions of gas molecules.
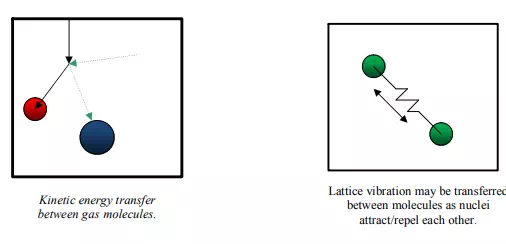
Solids, on the other hand, have atoms/molecules which are more closely packed which cannot move as freely as in gases. Hence, they cannot effectively transfer energy through these same mechanisms. Instead, solids may exhibit energy through vibration or rotation of the nucleus. Hence, the energy transfer is typically through lattice vibrations. Another important mechanism in which materials maintain energy is by shifting electrons into higher orbital rings. In the case of electrical conductors the electrons are weakly bonded to the molecule and can drift from one molecule to another, transporting their energy in the process. Hence, flow of electrons, which is commonly observed in metals, is an effective transport mechanism, resulting in a correlation that materials which are excellent electrical conductors are usually excellent thermal conductors