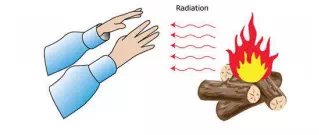
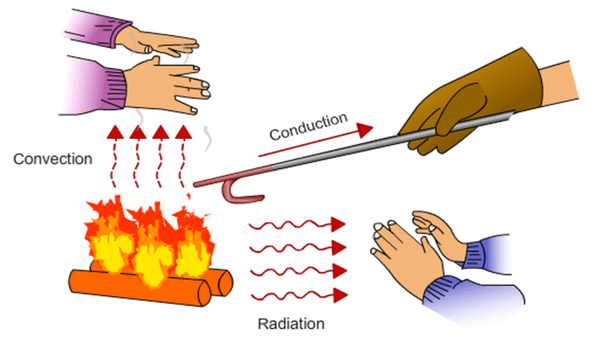
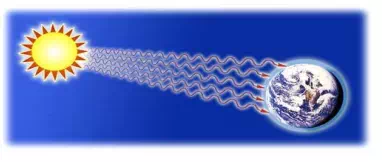
So we’ve learned that conduction moves heat easiest through solids, and convection moves heat through liquids and gases. So how does the heat from the Sun get to Earth? There are no molecules in space! And how do you feel the heat from a campfire, even if you’re sitting several feet away? The answer is radiation.
Radiation is how heat moves through places where there are no molecules. Radiation is actually a form of electromagnetic energy. Remember we learned that electromagnetic energy moves in waves? Well, radiation is heat moving in waves. Radiation does NOT need molecules to pass the energy along. All objects radiate heat, but some radiate much more heat than others.
The biggest source of radiation is the Sun – it sends a HUGE amount of heat to Earth through electromagnetic waves. (Last weekend, at the beach, I could definitely feel the wonderful heat radiation from the Sun. I guess that’s why I got a sunburn. Oops! A little too much radiation!)
Light bulbs radiate heat. Try it! Hold your hand a few inches away from a light bulb. You can feel the heat, right? In fact, a good way to remember radiation is that it is how you can feel heat without touching it. Heat passes through the empty space until it reaches your hand. That’s radiation! A fire is another example of radiation. Even YOU are an example. Your body gives off heat! (That’s why a classroom gets warm when there are a lot of people sitting in it.)