Hit Consider the entire sequence of elementary steps comprising a surface-catalyzed reaction: adsorption ofreactant(s), surface reaction(s), and finally desorption ofproduct(s). If the surface is considered uniform (i.e., all surface sites are identical kinetically and thermodynamically), and there are negligible interactions between adsorbed species, then derivation of overall reaction rate equations is rather straightforward. For example, the reaction of dinitrogen and dihydrogen to form ammonia is postulated to proceed on some catalysts according to the following sequence of elementary steps:
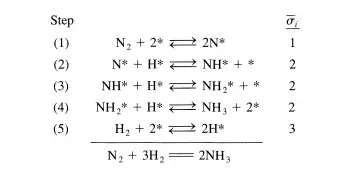
where (ii is the stoichiometric number of elementary step i and defines the number of times an elementary step must occur in order to complete one catalytic cycle according to the overall reaction. In the sequence shown above, a z = 2 means that step 2 must occur twice for every time that a dinitrogen molecule dissociately adsorbs in step 1. The net rate of an overall reaction can now be written in terms of the rate of anyone of the elementary steps, weighted by the appropriate stoichiometric number:

The final form of a reaction rate equation from Equation (5.3.1) is derived by repeated application of the steady-state approximation to eliminate the concentrations of reactive intermediates. In many cases, however, the sequence of kinetically relevant elementary steps can be reduced to two steps (M. Boudart and G. Djega-Mariadassou, Kinetics of Heterogeneous Cataiytic Reactions, Princeton University Press, Princeton, 1984, p. 90). For example, the sequence given above for ammonia synthesis can be greatly simplified by assuming step I is rate-determining and all other steps are nearly equilibrated. The two relevant steps are now:

It must be emphasized that step 2 is not an elementary step, but a sum of all of the quasi-equilibrated steps that must occur after dinitrogen adsorption. According to this abbreviated sequence, the only species on the surface of the catalyst of any kinetic relevance is N*. Even though the other species (H*, NH*, etc.) may also be present, according to the assumptions in this example only N* contributes to the site balance:
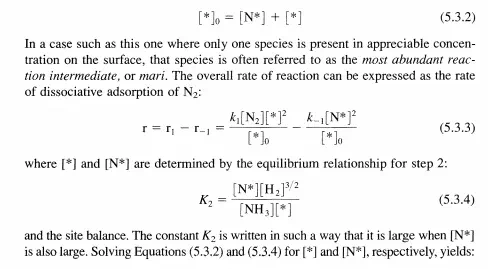
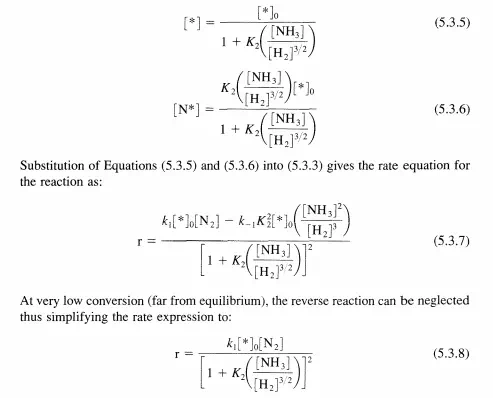
Ruthenium has been investigated by many laboratories as a possible catalyst for ammonia synthesis. Recently, Becue et al. [T. Becue, R. J. Davis, and 1. M. Garces, J. Catat., 179 (1998) 129] reported that the forward rate (far from equilibrium) of ammonia synthesis at 20 bar total pressure and 623 K over base-promoted ruthenium metal is first order in dinitrogen and inverse first order in dihydrogen. The rate is very weakly inhibited by ammonia. Propose a plausible sequence of steps for the catalytic reaction and derive a rate equation consistent with experimental observation.