Sintering, the welding together of small particles of metal by applying heat below the melting point. The process may be used in steel manufacturing—to form complex shapes, to produce alloys, or to work in metals with very high melting points. In a steel-sintering plant a bed of powdered iron ore, mixed with coke or anthracite, is ignited by a gas burner and then moved along a traveling grate. Air is drawn down through the grate to produce downdraft combustion. As the bed moves forward, a very high heat (1,325°–1,500° C [2,400°–2,700° F]) is generated that converts the tiny particles into lumps about 2.5 cm (1 inch) in diameter suitable for burning in the blast furnace to convert them to steel. Sintering is also used in the preliminary molding of ceramic or glass powders into forms that can then be permanently fixed by firing.
The driving force in sintering is decreasing surface energy; as the sintering proceeds, adjacent particles partially coalesce owing to viscous flow (as in glass) or to diffusion processes (as in crystalline materials), and consequently the total surface area decreases. The result is improved mechanical and physical properties of the material.
Diffusion
Diffusion, process resulting from random motion of molecules by which there is a net flow of matter from a region of high concentration to a region of low concentration. A familiar example is the perfume of a flower that quickly permeates the still air of a room.
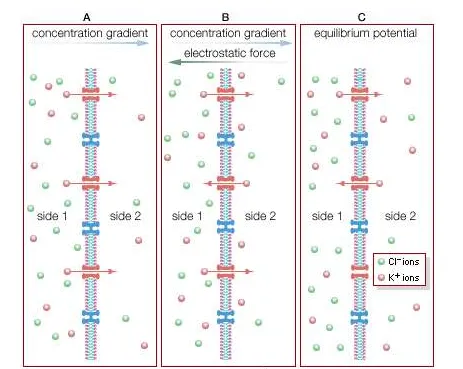
ion diffusion across a semipermeable membraneDiffusion of ions across a semipermeable membrane. (A) A high concentration of KCl is placed on side 1, opposite a semipermeable membrane from a low concentration. The membrane allows only K+ to diffuse, thereby establishing an electrical potential difference across the membrane. (B) The separation of charge creates an electrostatic voltage force, which draws some K+ back to side 1. (C) At equilibrium, there is no net flux of K+ in either direction. Side 1, with the higher concentration of KCl, has a negative charge compared with side 2.
Heat conduction in fluids involves thermal energy transported, or diffused, from higher to lower temperature. Operation of a nuclear reactor involves the diffusion of neutrons through a medium that causes frequent scattering but only rare absorption of neutrons.
The rate of flow of the diffusing substance is found to be proportional to the concentration gradient. If j is the amount of substance passing through a reference surface of unit area per unit time, if the coordinate x is perpendicular to this reference area, if c is the concentration of the substance, and if the constant of proportionality is D, then j = −D(dc/dx); dc/dx is the rate of change of concentration in the direction x, and the minus sign indicates the flow is from higher to lower concentration. D is called the diffusivity and governs the rate of diffusion.


Comments are closed