Coal gasification, any process of converting coal into gas for use in illuminating and heating. The first illuminating gas was manufactured from coal in England in the late 18th century by the process of carbonization or destructive distillation, heating coal in the absence of air, leaving a residue of coke as a by-product. Such coal gas was widely used for street lighting and home illumination until gaslight was displaced by electricity, and for heating and gas appliances until coal gas was displaced by natural gas. Processes for making lower grade industrial producer gas without coke by-products were developed in the mid-19th century, but competition from natural gas gradually eliminated such production. Increasing shortages of natural gas in the 1970s and ’80s led to the exploration of both new and old methods for producing gas from coal, among them a process developed in the 1870s in which coal is pulverized and mixed with oxygen and steam at high temperatures; similar methods using either air or carbon dioxide instead of oxygen; and processes exposing coal to hydrogen at high temperatures in the presence of a catalyst.
Coal
Coal, one of the most important primary fossil fuels, a solid carbon-rich material that is usually brown or black and most often occurs in stratified sedimentary deposits.

bituminous coal
Coal is defined as having more than 50 percent by weight (or 70 percent by volume) carbonaceous matter produced by the compaction and hardening of altered plant remains—namely, peat deposits. Different varieties of coal arise because of differences in the kinds of plant material (coal type), degree of coalification (coal rank), and range of impurities (coal grade). Although most coals occur in stratified sedimentary deposits, the deposits may later be subjected to elevated temperatures and pressures caused by igneous intrusions or deformation during orogenesis (i.e., processes of mountain building), resulting in the development of anthracite and even graphite. Although the concentration of carbon in Earth’s crust does not exceed 0.1 percent by weight, it is indispensable to life and constitutes humankind’s main source of energy.
History Of The Use Of Coal
In ancient times
The discovery of the use of fire helped to distinguish humans from other animals. Early fuels were primarily wood (and charcoal derived from it), straw, and dried dung. References to the early uses of coal are meagre. Aristotle referred to “bodies which have more of earth than of smoke” and called them “coal-like substances.” (It should be noted that biblical references to coal are to charcoal rather than to the rock coal.) Coal was used commercially by the Chinese long before it was used in Europe. Although no authentic record is available, coal from the Fushun mine in northeastern China may have been employed to smelt copper as early as 1000 BCE. Stones used as fuel were said to have been produced in China during the Han dynasty (206 BCE–220 CE).
In Europe
Coal cinders found among Roman ruins in England suggest that the Romans were familiar with coal use before 400 CE. The first documented proof that coal was mined in Europe was provided by the monk Reinier of Liège, who wrote (about 1200) of black earth very similar to charcoal used by metalworkers. Many references to coal mining in England and Scotland and on the European continent began to appear in the writings of the 13th century. Coal was, however, used only on a limited scale until the early 18th century, when Abraham Darby of England and others developed methods of using in blast furnaces and forges coke made from coal. Successive metallurgical and engineering developments—most notably the invention of the coal-burning steam engine by James Watt—engendered an almost insatiable demand for coal.
In the New World
Up to the time of the American Revolution, most coal used in the American colonies came from England or Nova Scotia. Wartime shortages and the needs of the munitions manufacturers, however, spurred small American coal-mining operations such as those in Virginia on the James River near Richmond. By the early 1830s mining companies had emerged along the Ohio, Illinois, and Mississippi rivers and in the Appalachian region. As in European countries, the introduction of the steam locomotive gave the American coal industry a tremendous impetus. Continued expansion of industrial activity in the United States and in Europe further promoted the use of coal.
Modern Utilization
Coal as an energy source
Coal is an abundant natural resource that can be used as a source of energy, as a chemical source from which numerous synthetic compounds (e.g., dyes, oils, waxes, pharmaceuticals, and pesticides) can be derived, and in the production of coke for metallurgical processes. Coal is a major source of energy in the production of electrical power using steam generation. In addition, gasification and liquefaction of coal produce gaseous and liquid fuels that can be easily transported (e.g., by pipeline) and conveniently stored in tanks. After the tremendous rise in coal use in the early 2000s, which was primarily driven by the growth of China’s economy, coal use worldwide peaked in 2012. Since then coal use has experienced a steady decline, offset largely by increases in natural gas use
Conversion
In general, coal can be considered a hydrogen-deficient hydrocarbon with a hydrogen-to-carbon ratio near 0.8, as compared with a liquid hydrocarbons ratio near 2 (for propane, ethane, butane, and other forms of natural gas) and a gaseous hydrocarbons ratio near 4 (for gasoline). For this reason, any process used to convert coal to alternative fuels must add hydrogen (either directly or in the form of water).
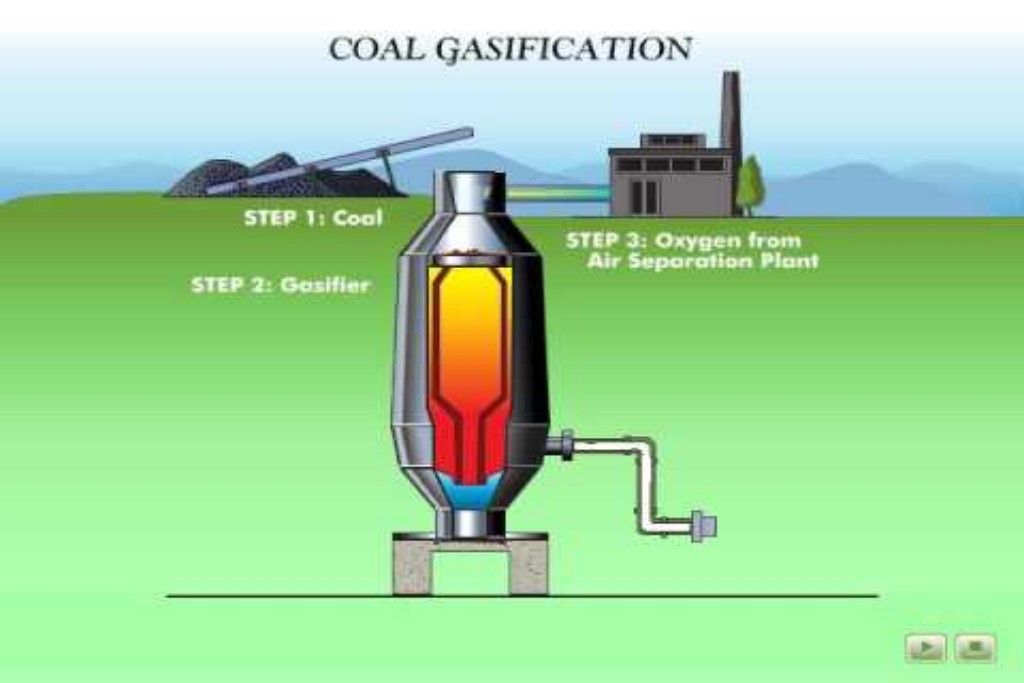
Gasification refers to the conversion of coal to a mixture of gases, including carbon monoxide, hydrogen, methane, and other hydrocarbons, depending on the conditions involved. Gasification may be accomplished either in situ or in processing plants. In situ gasification is accomplished by controlled, incomplete burning of a coal bed underground while adding air and steam. The gases are withdrawn and may be burned to produce heat or generate electricity, or they may be used as synthesis gas in indirect liquefaction or the production of chemicals.
Coal liquefaction—that is, any process of turning coal into liquid products resembling crude oil—may be either direct or indirect (i.e., by using the gaseous products obtained by breaking down the chemical structure of coal). Four general methods are used for liquefaction: (1) pyrolysis and hydrocarbonization (coal is heated in the absence of air or in a stream of hydrogen), (2) solvent extraction (coal hydrocarbons are selectively dissolved and hydrogen is added to produce the desired liquids), (3) catalytic liquefaction (hydrogenation takes place in the presence of a catalyst—for example, zinc chloride), and (4) indirect liquefaction (carbon monoxide and hydrogen are combined in the presence of a catalyst).


Comments are closed