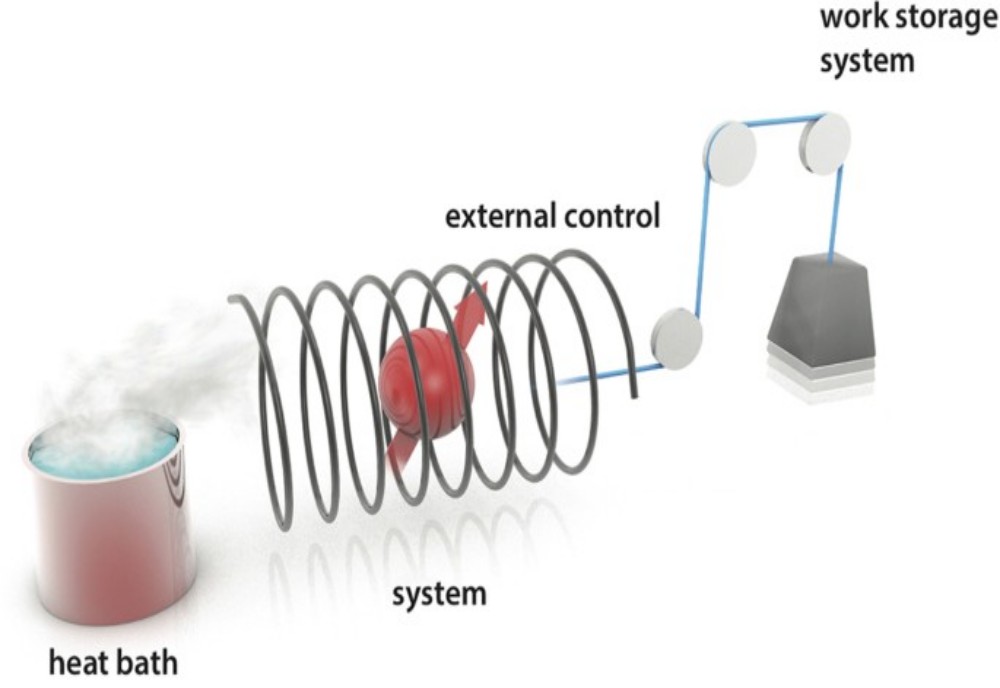
Although the answer to the simple question ‘what is temperature?’ seems obvious at first sight, it is surprisingly difficult to answer to everyone’s satisfaction. In fact, it is generally easier to state the corollary, ‘a body has a higher temper- ‘Corollary’ means a deduction following on from another, related, fact or series of facts. ature if it has more energy, and a lower temperature if it has less energy’. We have been rather glib so far when using words such as ‘heat’ and ‘temperature’, and will be more careful in future. Heat is merely one way by which we experience energy. Everything contains energy in various amounts, although the exact quantity of the energy is not only unknown but unknowable. Much of the time, we, as physical chemists, will be thinking The word ‘thermochemistry’ has two roots: thermo, meaning ‘temperature or energy’, and chemistry, the science of the combination of chemicals.
We see how ‘thermochemistry’ studies the energy and temperature changes accompanying chemical changes. about energy and the way energetic changes accompany chemical changes (i.e. atoms, ions or whole groups of atoms combine, or add, or are being lost, from molecules). While the total energy cannot be known, we can readily determine the changes that occur in tandem with chemical changes. We sometimes give the name thermochemistry to this aspect of physical chemistry. In practice, the concept of temperature is most useful when deterA body in ‘dynamic equilibrium’ with another exchanges energy with it, yet without any net change. mining whether two bodies are in thermal equilibrium. Firstly, we need to appreciate how these equilibrium processes are always dynamic, which, stated another way, indicates that a body simultaneously emits and absorbs energy, with these respective amounts of energy being equal and opposite.
Furthermore, if two bodies participate in a thermal equilibrium then we say that the energy emitted by the first body is absorbed by the second; and the first body also absorbs a similar amount of energy to that emitted by the second body. Temperature is most conveniently visualized in terms of the senses: we say something is hotter or is colder. The first thermometer for studying changes in temperature was devised in 1631 by the Frenchman Jean Rey, and comprised a length of water in a glass tube, much like our current-day mercury-in-glass thermometers but on a much bigger scale. The controlled variable in this thermometer was temperature T , and the observed variable was the length l of the water in the glass tube. Rey’s thermometer was not particularly effective because the density of water is so low, meaning that the volume of the tube had to be large. And the tube size caused an additional problem.
While the water expanded with temperature (as required for the thermometer to be effective), so did the glass encapsulating it. In consequence of both water and glass expanding, although the water expanded in a straightforward way with increasing temperature, the visible magnitude of the expansion was not in direct proportion to the temperature rise. Although we could suggest that a relationship existed between Scientists use the word ‘ideal’ to mean obeying the laws of science. the length l and the temperature T (saying one is a function of the other), we could not straightforwardly ascertain the exact nature of the function. In an ideal thermometer, we write the mathematical relationship, l = f (T ). Because Rey’s thermometer contained
water, Rey was not able to observe a linear dependence of l on T for his thermometer, so he could not write l = aT + b (where a and b are constants). For a more dense liquid, such as mercury, the relationship bet- ‘Narrow’ in this case means 50–70 ◦ C at most. ween l and T is linear – at least over a relatively narrow range of temperatures – so a viable mercury-in-glass thermometer may be constructed. But, because the temperature response is only linear over a narrow range of temperatures, we need to exercise caution.