The hydrocarbons or any carbon compounds containing more than one halogen atom (group 17 element of the modern periodic table) are known as polyhalogen compounds. Many of these compounds are useful in industry and agriculture. Some notable polyhalogen compounds are described below:
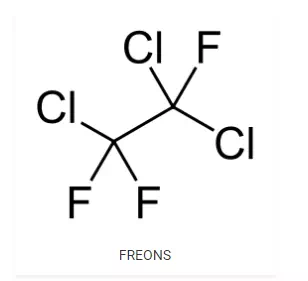
FREONS (CFCs)
· Freons are the chlorofluorocarbon compounds of methane and ethane. The chlorofluorocarbon compounds refer to the compounds having mainly carbon, fluorine and chlorine. Freons is the brand name for this group of compound coined by DuPont.
· They are extremely stable, unreactive, non-toxic, non-corrosive and easily liquefiable gases.
· Freon 12 or R-12 (CCl2F2) is one of the most common representatives of this group. It is manufactured from tetrachloromethane by Swarts reaction.
· These are usually produced for aerosol propellants, refrigeration and air conditioning purposes.
DDT (p, p’–Dichlorodiphenyltrichloroethane)
· It is a colorless,crystalline, tasteless and almost odorless organochloride known for its insecticidal
· It was the first chlorinated organic insecticides prepared in 1873. But in 1939, Paul Miller identified the different uses of DDT. Paul Muller was awarded the Nobel Prize in Medicine and Physiology in 1948 for this discovery.
· It became popular because of its effectiveness against the mosquito that spreads malaria and lice that carry typhus.
· But due to ill-effects of DDT such as chemical instability and fat solubility, it got banned in many countries
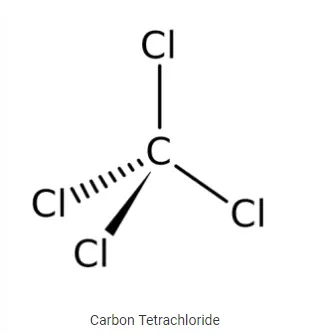
Carbon Tetra chloride (Tetrachloromethane)
· It is a colourless liquid with a “sweet” smell that can be detected at low levels. Molecular formula of carbon tetra chloride is CCl4.
· It is used in manufacturing of refrigerants, as cleaning agent and was also used a fire extinguisher.
· Medically, it is one of the most potenthepatotoxins (toxic to the liver), and is widely used in scientific research to evaluate hepatoprotective agents.
· When carbon tetrachloride is released into air, it rises in the atmosphere and depletes the ozone layer. Depletion of the ozone layer is believed to increase human exposure to ultraviolet rays, leading to increased skin cancer, eye diseases and disorders, and possible disruption of the immune system

Comments are closed