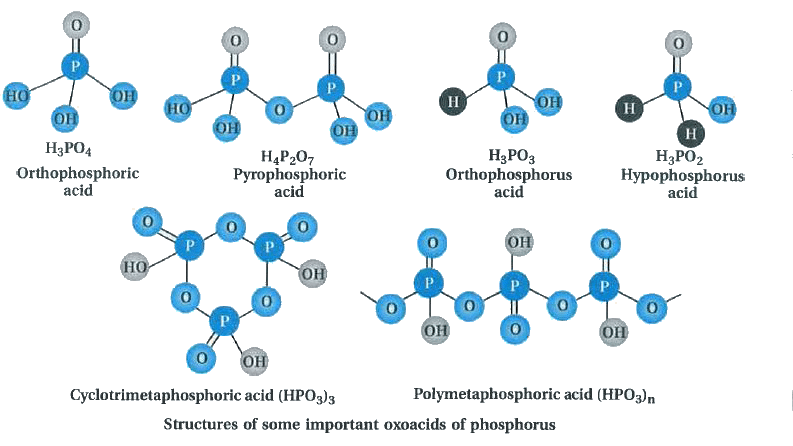
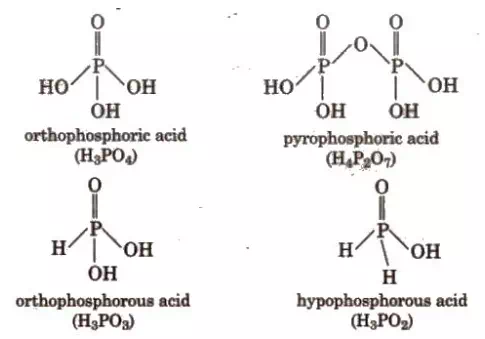
Oxoacids are basically acids that contain the element oxygen. As such, Phosphorus is known to form a number of oxoacids, for example: H3PO4, H3PO3, etc. In oxoacids of phosphorus, it is tetrahedrally surrounded by other atoms. Generally, all these acids are known to form at least one P=O bond and one P–OH bond.
P–P or P–H bonds are also found in addition to P=O bonds and P–OH bonds in oxoacids of phosphorus where the oxidation state of phosphorus is less than +5. These acids are generally seen to disproportionate to either lower and higher oxidation states. For instance, when phosphorous acid is heated, it gives phosphine and phosphoric acid.
4H3PO3 → 3H3PO4 + PH3
The P-H bonds in oxoacids cannot go through ionization to give H+ ions whereas the H atoms which are attached with oxygen in P-OH form are ionisable. Hence we can say that only the H atoms attached with oxygen cause basicity. As a result phosphorous acid, H3PO3 is dibasic due to the presence of two P-OH bonds whereas phosphoric acid, H3PO4 is tribasic due to the presence of three P-OH bonds. Oxoacids of phosphorus having P-H bonds have strong reducing properties. For instance: hypophosphorous acid containing two P-H bonds acts as a good reducing agent.
4 AgNO3 + 2H2O + H3PO2 → 4Ag + 4HNO3 + H3PO4
Few popular oxoacids of phosphorus
Phosphorus acid, H3PO3: Phosphorous acid is a diprotic acid that is, it ionizes two protons. It is better described with the structural formula HPO(OH)2 Phosphorous acid is prepared by hydrolysis of phosphorus trichloride with acid or steam.
PCl3 + 3 H2O → HPO(OH)2 + 3 HCl
Phosphoric acid, H3PO4: Phosphoric acid is a triprotic acid that is, it ionizes three protons. It is a non-toxic acid, when pure and is a solid at room temperature and pressure. Phosphoric acid is prepared by adding sulfuric acid to tricalcium phosphate rock:
Ca5(PO4)3X + 5 H2SO4 + 10 H2O → 3H3PO4 + 5 CaSO4.2H2O + HX
X can be F, Cl, Br and OH
Meta Phosphoric (HPO3)n
This acid is formed by warming orthophosphoric acid at around 850 K. Metaphosphoric acid exists as a cyclic trimer, polymer or cyclic tetramer but not as a monomer.
H3PO4 → HPO3 + H2O
Hypophosphoric Acid (H4P2O6)
Hypophosphoric acid is formed by conducting a controlled oxidation of red phosphorus with sodium chlorite. When the disodium salt of the acid is formed it then moves via cation exchanger that ultimately gives hypophosphoric acid. The acid is of tetrabasic nature.
2PH+ 2NaClO2 + 2H2O ———> Na2H2P2O6 + 2HCl
Na2H2P2O6 + 2H —–resin—–> H4P2O6 + 2Na – resin
Pyrophosphoric Acid (H4P2O7)
When orthophosphonic acid is heated at about 250oC it forms tetrabasic acid.
2H3PO4 ———> H4P2O7 + H2O
Orthophosphoric Acid (H3PO4)
This acid is formed when P4O10 is treated with bubbled water. Orthophosphoric Acid is basically a tribasic acid.
P4O10 + 6H2O ———-> 4H3PO4