Oxidation and Reduction in terms of Oxygen transfer
In early chemistry, oxidation and reduction were terms associated with oxygen. Oxidation meant gaining oxygen and Reduction meant losing oxygen. The term ‘reduction’ comes from Latin and means ‘-to lead back’. Therefore anything that leads back to the free metal state is referred to as a reduction reaction.
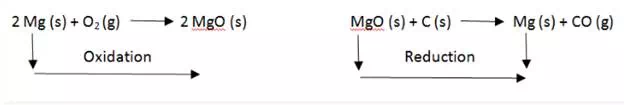
Magnesium undergoes both oxidation and reduction in reactions with different reactants.
Oxidation and Reduction in terms of Electron transfer
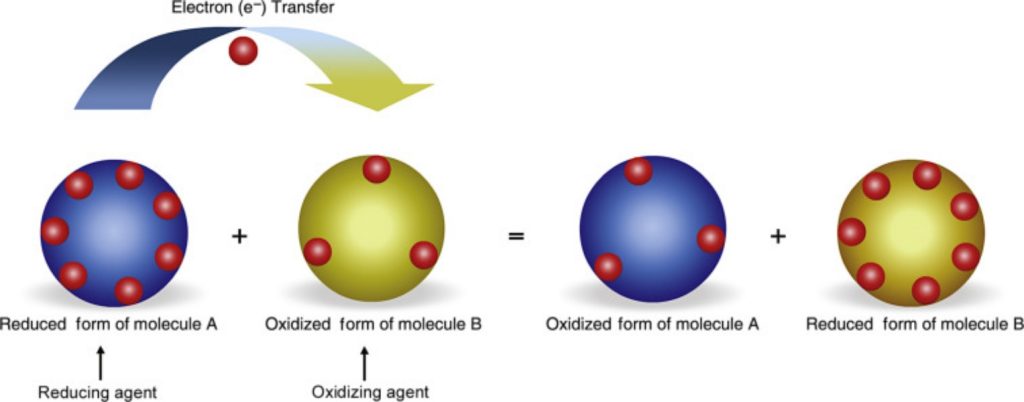
This is the most commonly used definition of oxidation and reduction and most widely applicable. In this case, Oxidation is the loss of electrons and Reduction is the gain of electrons. A very clever mnemonic to remember this concept is oil rig.
OIL RIG
Oxidation is loss Reduction is gain
Oxidation and Reduction reactions are always interlinked. Because electrons are neither created nor destroyed in a chemical reaction, oxidation and reduction always occur in pairs, it is impossible to have one without the other. In the below reaction Magnesium gets oxidized by losing two electrons to oxygen which gets reduced by accepting two electrons from magnesium.
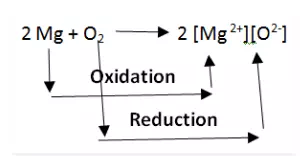
Since oxidation and reduction cannot occur individually, they as a whole are called ‘Redox Reactions’. The reactant that oxidizes the other reactants is called as the Oxidizing agent and reactant that reduces is called Reducing agent. There is quite some confusion about the aspect whether oxidizing agents accept or give away electrons.
The following steps can help you figure it out.
· An oxidizing agent oxidizes the other reactants
· This must mean that the oxidizing agent is getting reduced
· Oxidation is loss of electrons (OIL RIG)
· So an oxidizing agent must gain electrons
Oxides Of Nitrogen
Nitrogen reacts with oxygen to form a number of nitrogen oxides. It exhibits different oxidation states in its oxides, ranging from +1 to +5. Oxides of nitrogen having nitrogen in the higher oxidation state are more acidic than that in lower oxidation state. Some of the oxides of nitrogen are enlisted below:
Dinitrogen oxide, N2O
Dinitrogen oxide is a colourless non-flammable gaseous compound with neutral properties. It is commonly known as laughing gas. Dinitrogen oxide is prepared by the decomposition of ammonium nitrate under high temperature.
NH4NO3 → N2O + 2H2O
Nitrogen monoxide, NO:
Nitrogen monoxide is a colourless gaseous compound. It’s bonding structure includes an unpaired electron and it belongs to the class of heteronuclear diatomic molecules. Nitrogen monoxide is prepared by the reduction of dilute nitric acid with copper.
2 NaNO2 + 2 FeSO4 + 3 H2SO4 → Fe2(SO4)3 + 2NaHSO4 + 2H2O + 2 NO
Dinitrogen trioxide, N2O3:
Dinitrogen trioxide is a deep blue solid with an acidic nature. It is only isolable at low temperatures, i.e. in the liquid and solid phases. As the temperature increases, the equilibrium favours the formation of constituent gases. Dinitrogen trioxide is prepared by mixing equal parts of nitric oxide and nirogen dioxide and further cooling the mixture below −21 °C (−6 °F).
NO + NO2 → N2O3
Nitrogen dioxide, NO2:
Nitrogen dioxide is a reddish-brown toxic gas and has a characteristic sharp, biting odor and is a prominent air pollutant. It is acidic in nature having +4 oxidation state of nitrogen. Nitrogen dioxide is prepared by thermal decomposition of metal nitrates, for example lead nitrate:
2 Pb(NO3)2 → 2 PbO + 4 NO2 + O2
Dinitrogen tetroxide, N2O4:
Dinitrogen tetroxide is a colourless solid existing in equilibrium with nitrogen dioxide. It is a powerful oxidizer and is used as a reagent in the synthesis of many chemical compounds.
N2O4 ⇌ 2 NO2
Dinitrogen Pentoxide, N2O5:
Dinitrogen pentoxide is a colourless solid that sublimes slightly above room temperature. It is an unstable and potentially dangerous oxidizer and was used as a reagent, dissolved in chloroform for nitration. Dinitrogen pentoxide is prepared by dehydrating nitric acid (HNO3) with phosphorus (V) oxide:
P4O10 + 12 HNO3 → 4 H3PO4 + 6 N2O5