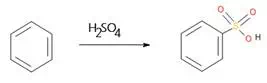
 Figure 1: Example of Aromatic Sulfonation Reaction
Figure 1: Example of Aromatic Sulfonation Reaction
Sulfonation may be defined as any chemical process by which the sulfonic acid group -SO2OH, or the corresponding salt or sulfonyl halide group (e.g., -SO2Cl), is introduced into an organic compound. The sulphonates thus produced are not just used in the dyestuffs industry, but also used as concrete additives, surfactants, corrosion inhibitors and much more.
Commercial sulphonation is divided into a batch and continuous type process. Each of these processes has its own advantages and disadvantages.
A batch process is a preferred process in case of sulphonation. Batch processes are mainly used when:
● Suitable for small scale production
● Suitable for processes where a range of different products or grades is to be produced in the same equipment
● Suitable for reactions requiring long reaction times
● Suitable for reactions with superior selectivity
What keeps sulfonation from being a green process?
Sulphonation reactions are mainly governed by heat and mass transfer capabilities of the process. This is because sulphonation reaction mixtures are viscous in nature. This inherently makes the process inefficient with respect to heat exchange and thus contributes to poor product quality. Other than this, the following are the aspects of the process that contribute to pollution and are hazardous to the environment.
● A solution of dilute H2SO4 is generated after sulphonation reaction is a hazardous pollutant and its disposal is a very difficult process. The cause of generation in almost all cases is an excess addition of sulfuric acid for sulfonation. Excess sulfuric acid is added to facilitate mixing due to a low solubility of a product in the reaction mixture and thereby completion of a reaction.
● Neutralization of dilute sulfuric acid generated after sulfonation. Neutralization generates a large quantity of solid waste (gypsum).
● For the production of sulfonated polymers a swelling agent such as chloro-hydrocarbon is used, which is harmful to the environment.
● By-product like sulfones produced which causes a decrease in the yield of the product.
● A conventional process for the preparation of aromatic sulfones consists in carrying out a sulfonation reaction of Friedel-Crafts type. The aromatic compound and an aryl sulfonating agent are reacted in the presence of a catalyst, which is generally aluminum chloride. However, the use of aluminum chloride exhibits numerous disadvantages. Aluminum chloride is a corrosive and irritating product, corrosive in nature, used in large amounts instead of stoichiometric amounts, separation of AlCl3 is difficult – time consuming and expensive and it cannot be recycled.
Other than its harmful effects on the environment, sulphuric acid like many chemicals is dangerous to handle and needs rigorous precautionary measures. However, many incidents have been reported that included people being burned from it or even have got killed. A list of such events can be found atOSHA’s website for accident search. The Department of Health of New York State also furnished a report of sulphuric acid spills in the state. According to its report, most sulphuric acid spills were reported during manufacture (38%), transport (21%), and at power generation facilities (15%) where the acid is used for cleaning boilers and piping by dissolving mineral deposits. Known causes for acid events reported to the study were equipment failure (47%), and human error (15%).
Such incidents can be avoided by proper inventory management, preparation of clean-up materials in the events of spills, use of safety equipment, know-how of safety procedures, use of MSDS (material safety data sheet) and basic chemical hygiene. This applies both at the laboratory as well as industrial level. Workers should be made aware of the accidents have had happened with even the most experienced scientists. Sometimes even a basic check on outdated fire extinguishers or good laboratory practices such incident reporting and preparatory measures such as first-aid kit or attitude such as ‘it will not happen to me’ can mean the difference between life and death. Young engineers or chemists may feel like an odd-man out when their seniors do not follow safety guidelines and choose to do the same under pressure. Workers, irrespective of their seniority, should not be ashamed to take care of themselves and their fellow workers.


Comments are closed