Stoichiometry:
When chemical reactions occur, in contrast with physical changes of material such as evaporation or dissolution, you want to be able to predict the mass or moles required for the reaction(s), and the mass or moles of each species remaining after the reaction has occurred. Reaction stoichiometry allows you to accomplish this task. The word stoichiometry (stoi-ki-om-e-tri) derives from two Greek words: stoicheion (meaning “element”) and metron (meaning “measure”). Stoichiometry provides a quantitative means of relating the amount of products produced by chemical reactions to the amount of reactants. The following expressions are widely used in stoichiometry:
1. Stoichiometric coefficients:
The numbers that are precede the chemical substances involved in the chemical reaction equation are known as ” stoichiometric coefficients”. These coefficients represent quantity of any reactant that is theoretically required for complete conversion of other reactants.
2. Stoichiometric ratios:
The ratio between any stoichiometric coefficients in a balanced chemical equation is known as ” stoichiometric ratio”.
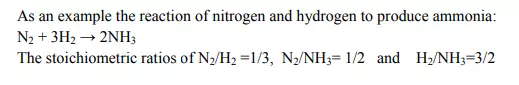
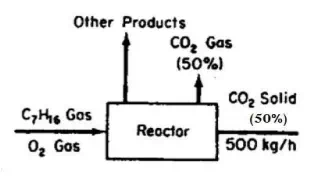
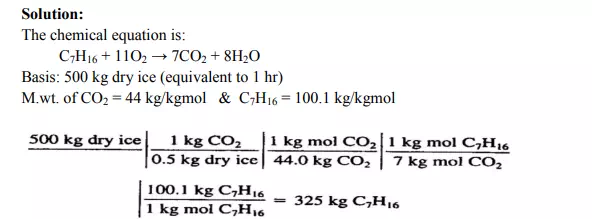
Use of the Chemical Equation to Calculate the Mass of Reactants Given the Mass of Products In the combustion of heptane, CO2 is produced. Assume that you want to produce 500 kg of dry ice per hour, and that 50% of the CO2 can be converted into dry ice, as shown in Figure E9.2. How many kilograms of heptane must be burned per hour?