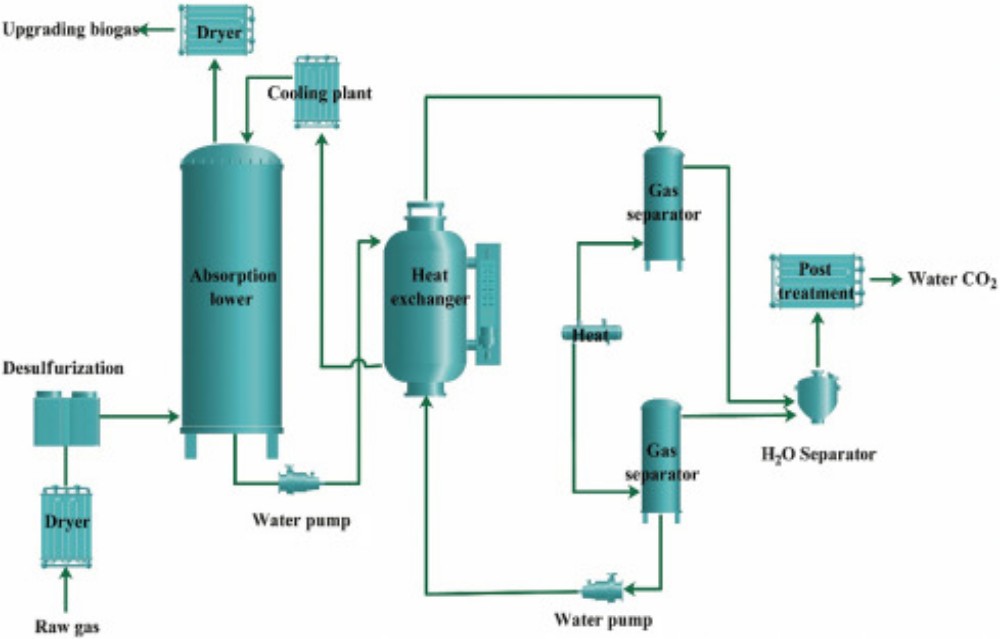
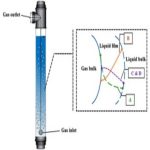
Operations in which one or more components of a gas phase are absorbed into a liquid phase are common throughout the chemical process industries and frequently serve to achieve desired reactions among components in the two phases (Lee & Tsui, 1999). Such operations are often called reactive absorption because of the combination of reaction and absorptive mass transport. There are a number of cases in which a gas, on absorption, reacts chemically with a component of the liquid phase. In such processes, the conditions in gas phase are similar to those of an entirely physical absorption process, but in the liquid phase, there is a liquid film followed by a reaction zone. As an example, in the absorption of carbon dioxide by caustic soda, the carbon dioxide reacts directly with the caustic soda. An advantage of absorption plus reaction is the increase in the mass-transfer coefficient. This may be due to a greater effective interfacial area. The process hydrodynamics can also be directly involved via correlations for the hold-up, pressure drop, and mass transfer coefficients, etc.
Absorption-Reaction Model
The fundamental relations governing simultaneous diffusion and chemical reaction of a dissolved species have been reviewed by Danckwerts (1970). For one-dimensional diffusion of a single species (A) with diffusivity independent of concentration.

The reaction rate term r, is generally a function of solute concentration and of one or more liquid reactant concentrations. If these reactant concentrations vary appreciably, continuity equations for each reactant must be solved simultaneously with Equation (4.47) to obtain the solute concentration profile. An increase in the rate of absorption caused by reaction is a result of a concentration drop in the bulk liquid phase.