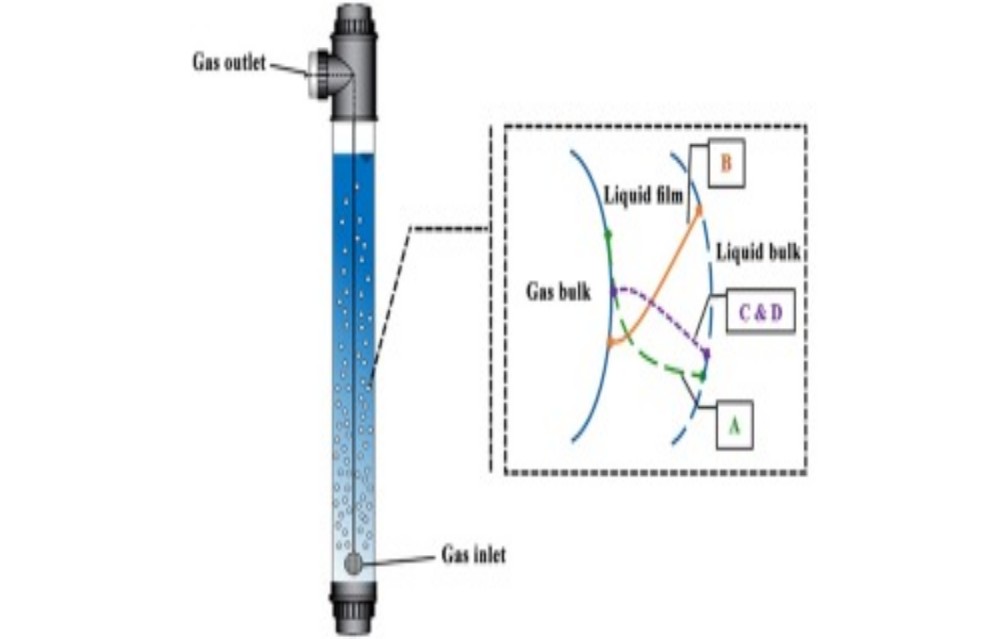
The bulk concentration becomes zero when the absorption process is accompanied by a fast irreversible first-order reaction. Then as per film theory the following balance can be written:



The Equation (4.55) indicates that the absorption rate is independent of the mass transfer coefficient and therefore the hydrodynamic conditions prevailing at the interface. The Equation (4.55) can be used to estimate the interfacial area (Si) in gas-liquid reactor as

where A0 n & is the initial molar flow and U is the overall gas phase conversion. Therefore the specific interfacial area (the interfacial area per unit volume of liquid (VL) in the reactor) can be expressed as

The ratio of specific absorption rate (RA) to the * LCA k is called enhancement factor of absorption from the diffusion regime. The Equation (4.58) also forms the basis for the calculation of absorption rate referred to as the liquid volume (VL):