We have learned that heat is the energy that makes molecules move. Molecules with more heat energy move faster, and molecules with less heat energy move slower. We also learned that as molecules heat up and move faster, they spread apart and objects expand (get bigger). This is called thermal expansion.
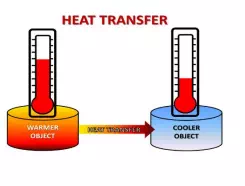
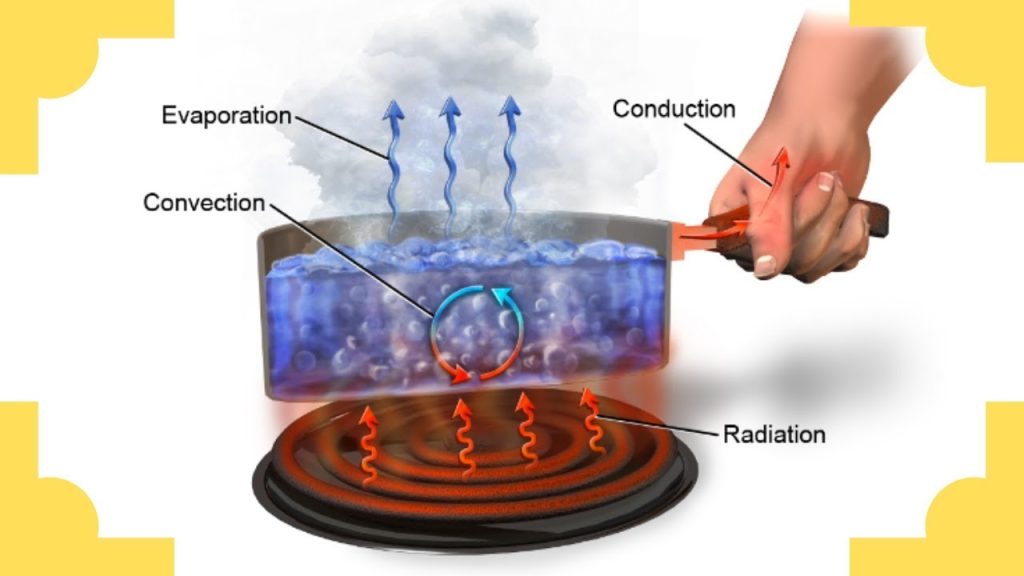
Heat is always moving! If you have two objects or substances that are different temperatures, heat will always move OUT of the warmer object or substance, and INTO the cooler object or substance. This heat transfer will continue until the objects are the same temperature. So how, exactly, does heat move out of one thing and into another thing? This is called heat transfer. (Remember, we learned that energy transfer is when energy moves from one thing or place to another, but the energy type stays the same). Heat can transfer (or move) in 3 ways: conduction, convection, and radiation. As you read about the three types of heat transfer, pay attention to:
· What the heat is moving through (solids, liquids and gases, or empty space)
· How the heat is being transferred (touch, currents, or waves)
Conduction
Last weekend, I went to the beach. I was walking barefoot on the soft, cool grass. When I got to the sand, I noticed that my feet were burning! Ouch! This is an example of conduction.
Conduction is how heat transfers through direct contact with objects that are touching. Any time that two objects or substances touch, the hotter object passes heat to the cooler object. (That hot sand passed the heat energy right into my poor feet!) Think of a row of dominoes that are all lined up. When you push the first domino, it bumps into the second one, which bumps into the third one…all the way down the line.
Heat conduction is like the dominoes. Imagine that you place one end of a metal pole into a fire. The molecules on the fire end will get hot. Each hot molecule will pass the heat along to the molecule next to it, which will pass the heat along to the next molecule, and so on. Before you know it, the heat has traveled all the way along the metal pole until it reaches your hand.

Some materials are better conductors than others. That’s because some materials are able to pass (conduct) heat more easily. Metals are great conductors. That’s why metal objects get hot so easily. Plastic and wood are poor conductors. They will still get hot, but it takes a lot longer for them to pass the heat from molecule to molecule
Comments are closed