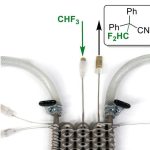
At the end of the summer, Steve Johnson became one of the legions of Americans curious about a new weight-loss drug called Wegovy (semaglutide). The medical device salesperson had been fasting intermittently and was exercising more, but the roughly 60 lb (27 kg) he wanted to lose weren’t coming off.
He researched Novo Nordisk’s data for the drug, was impressed by the average 15% weight loss seen in clinical trial participants, and in August called his doctor.
The once-weekly shot of Wegovy might be the boost he needs, he told his physician. That is, if he could get his hands on it.
“It’s been back-ordered,” Johnson says. “At first, it was a couple of weeks, and then they drew it out, and then I heard the end of September.”
Novo Nordisk was unprepared for the demand for Wegovy, which the US Food and Drug Administration approved in early June. Social media groups are filled with people sharing tips on places that have doses in stock and people lamenting when they can’t find the drug. Company officials say it might take until the end of the year to clear the backlog.
Many industry, government, and academic scientists who study metabolism and appetite say the clamor for Wegovy demonstrates the need for safer and more-effective weight-loss drugs. In a field plagued by drugs with dangerous side effects and product recalls, doctors have been hesitant to prescribe these products—and companies have been nervous about investing in developing new ones. The emergence of Wegovy and a handful of other drugs over the past few years coincides with a shift in the medical complex’s and pharmaceutical industry’s description of people who have obesity, a controversial word that is defined by the US Centers for Disease Control and Prevention as anyone with a body mass index (BMI) of 30 or higher. In the past, obesity was framed as a behavioral issue. Today, it is seen by many as a chronic disease that, because of links to other conditions like diabetes, heart disease, and high blood pressure, warrants continuous treatment.
“The problem with obesity is that it’s a chronic illness and that it’s relapsing,” says John Sharretts, an endocrinologist who is the deputy director of the FDA’s Division of Diabetes, Lipid Disorders, and Obesity. “People lose weight and they regain and it’s complicated. The goal of weight loss is the long-term reduction of excess fat with the purpose of reducing those health consequences.”
Wegovy is one of five drugs on the market for what US regulators call “chronic weight management.” Pharma companies’ pipelines, once devoid of anything related to weight loss, are now flush with drug candidates.
“It’s actually quite interesting to watch the field slowly go back into the obesity area, which was clearly for quite a few years a no-go zone, particularly with Big Pharma,” says Philipp Scherer, a metabolism scientist at the University of Texas Southwestern Medical Center.
The financial potential of a safe and effective weight-loss drug is staggering. About 42% of people in the US meet the CDC’s definition of obesity. And people with lower BMIs who have metabolic diseases are eligible for either Wegovy or a shorter-acting version of the drug called Saxenda (liraglutide) that was approved in 2014. Chronic weight management means years, if not decades, of treatment. Saxenda, which is taken daily and typically offers more modest weight loss, brought in more than $900 million in sales in 2020.
But this surge in development runs counter to another shift—one that says being fat is not a disease. A 2016 study from the University of California, Los Angeles, suggests that 29% of people who are medically defined as obese are otherwise metabolically healthy. Ragen Chastain, a public speaker and writer on the subjects of fat discrimination and the pathologizing of weight, says fat is a normal state of being and that calling it a disease is stigmatizing.
“This has become something that’s driven tremendous profit. In terms of what it’s done for the community—people who have been labeled—it’s driven stigma, oppression,” she says.

DECADES OF IMPERFECT PILLS
The history of weight-loss drug development is a tumultuous one. From its earliest days, products have been marred by side effects ranging from high blood pressure to death.
The problems started in the 1930s, when 2,4-dinitrophenol (DNP) became one of the first chemicals used for weight loss. DNP is a mitochondrial uncoupler that works by interfering with the reactions that create and store energy in our bodies. With no place to go, the energy is released as heat, leading to weight loss.
It can also lead to uncontrolled increases in body temperature, and the drug has never been approved by modern-day regulatory authorities. People can still buy DNP on the internet, though, and between 2001 and 2010, a dozen people died after taking it, including a 28-year-old man using the drug as a bodybuilding tool. His internal body temperature peaked at 106 °F (41 °C).
In the 1960s, FDA-approved weight-loss drugs were all derivatives of amphetamine, which suppresses appetite. One of those pills, phentermine, is still widely prescribed, says physician Daniel Bessesen, a researcher at the University of Colorado Anschutz Medical Campus. People taking phentermine on average lose between 5 and 10% of their starting weight. For people whose insurance won’t cover drugs like Wegovy, it’s the best available option, Bessesen says.
But this class of drugs can be addictive and cause dangerously high blood pressure, so the drugs can be taken for only a few weeks at a time.
The 1970s and 1980s were a long dry spell for new weight-loss drugs. That ended in 1996, when the FDA approved dexfenfluramine as a stronger version of the previously approved weight-loss drug fenfluramine. Both drugs were part of a class of appetite suppressants called serotonergic anorectics, which work by lowering the amount of serotonin in the brain. Doctors had previously paired phentermine with fenfluramine, in an infamous combo dubbed fen-phen.
Then came reports of heart valve damage in people taking fen-phen. In 1997, the FDA pulled fenfluramine and dexfenfluramine off the market, marking the beginning of a merry-go-round of regulatory rejections, approvals, and withdrawals for weight-loss drugs. Meridia (sibutramine) was approved by the FDA in 1997 and then withdrawn from the market in 2010 because of side effects that included an increased risk of heart attack. Rimonabant, the first blocker of cannabinoid receptor 1 for weight loss, was approved in Europe in 2006 but pulled 2 years later after the drug was linked to thoughts of suicide. Belviq (lorcaserin), a small molecule that stimulates a serotonin receptor, was pulled in 2020 after 8 years on the market because it was shown to increase the risk of cancer.
Nadia Ahmad, the medical director for Eli Lilly and Company’s obesity product development, says this long string of safety issues and product failures is why the field has been considered such a gamble for pharma companies. “You have one drug that goes off the market for whatever reason, and it really shuts down for like a good decade any further pharmaceutical development,” she says.
The troubled history has necessarily raised the regulatory bar for weight-loss drugs. Today, on top of requiring evidence that a new drug can allow someone to maintain a weight loss of 5–10% of their starting weight for a year, the FDA also wants to see longer-term studies proving it is safe.
Safety also became more critical as the field shifted from acute to chronic use, a transition that began in 1999 with a new drug called Xenical (orlistat). The pill works by blocking fat from being absorbed by the body. While it can cause constant diarrhea for some, it was the first of several drugs allowed to be taken long term.
Older weight-loss drugs not only were prone to dangerous side effects but also didn’t work as well as people wanted. Losses were modest—around 5–10%—and people typically regained weight as soon as they stopped taking the drugs. This weight cycling is a source of mental health issues, as people internalize the failure of those products, Chastain says.
“There is something wrong with an intervention that has the exact opposite of the intended effect the majority of the time,” she says. “What the weight-loss industry has done brilliantly is to take credit for the first part of that biological response where people lose weight short term and blame their clients and get their clients to blame themselves for the second part of the same biological response—when they gain the weight back.”