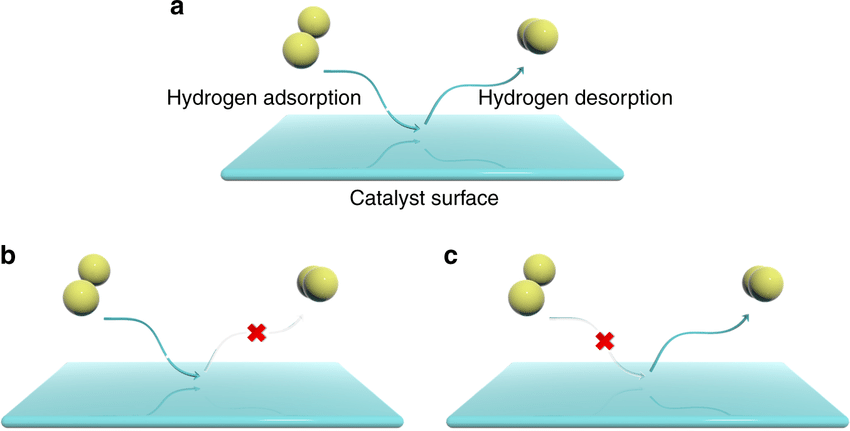
The necessary first step in a heterogeneous catalytic reaction involves activation of a reactant molecule by adsorption onto a catalyst surface. The activation step implies that a fairly strong chemical bond is formed with the catalyst surface. This mode of adsorption is called chemisorption, and it is characterized by an enthalpy change typically greater than 80 kJ mol- J and sometimes greater than 400 kJ mol- J.
Since a chemical bond is formed with the catalyst surface, chemisorption is specific in nature, meaning only certain adsorbate-adsorbent combinations are possible. Chemisorption implies that only a single layer, or monolayer, of adsorbed molecules is possible since every adsorbed atom or molecule forms a strong bond with the surface. Once the available surface sites are occupied, no additional molecules can be chemisorbed. Every molecule is capable of weakly interacting with any solid surface through van der Waals forces.
The enthalpy change associated with this weak adsorption mode, called physisorption, is typically 40 kJ mol- J or less, which is far lower than the enthalpy of chemical bond formation. Even though physisorbed molecules are not activated for catalysis, they may serve as precursors to chemisorbed molecules. More than one layer of molecules can physisorb on a surface since only van der Waals interactions are involved. The number of physisorbed molecules that occupy a monolayer on the surface of any material can be used to calculate its geometric surface area if the cross-sectional area of the adsorbate molecule is known.
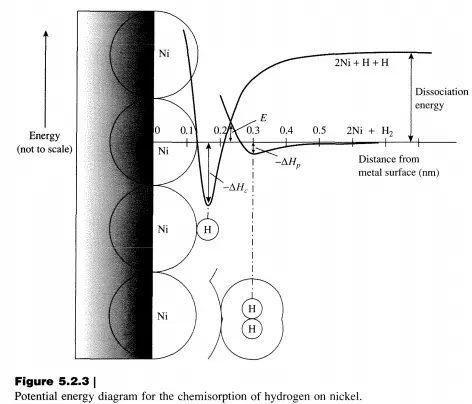
The potential energy diagram for the chemisorption of hydrogen atoms on nickel is schematically depicted in Figure 5.2.3. As molecular hydrogen approaches the surface, it is trapped in a shallow potential energy well associated with the physisorbed state having an enthalpy ofphysisorption flHp
• The deeper well found closer to the surface with enthalpy flHc is associated with the hydrogen atoms chemisorbed on nickel. There can be an activation barrier to chemisorption, E, which must be overcome to reach a chemisorbed state from the physisorbed molecule. Since molecular hydrogen (dihydrogen) is dissociated to form chemisorbed hydrogen atoms, this phenomenon is known as dissociative chemisorption. Because rates of heterogeneous catalytic reactions depend on the amounts of chemisorbed molecules, it is necessary to relate the fluid phase concentrations of
reactants to their respective coverages on a solid surface. For simplicity, the following discussion assumes that the reactants are present in a single fluid phase (i.e., either liquid or gas), all surface sites have the same energetics for adsorption, and the adsorbed molecules do not interact with each other. The active site for chemisorption on the solid catalyst will be denoted by *, with a surface concentration (*]. The adsorption of species A can then be expressed as:
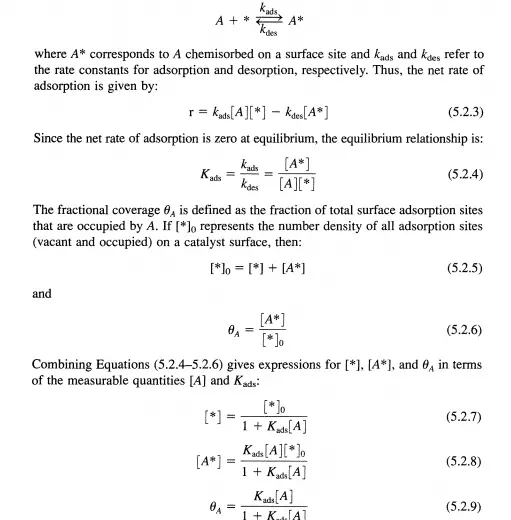
When more than one type of molecule can adsorb on the catalyst surface, the competition for unoccupied sites must be considered. For the adsorption of molecule A in the presence of adsorbing molecule B, the site balance becomes

where the equilibrium constants are now associated with adsorption/desorption of either A or B