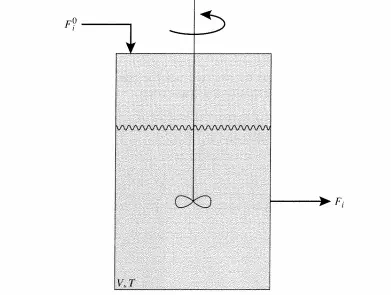
The ideal reactor for the direct measurement of reaction rates is an isothermal, constant pressure, flow reactor operating at steady-state with complete mixing such that the composition is uniform throughout the reactor. This ideal reactor is frequently called a stirred-tank reactor, a continuous flow stirred-tank reactor (CSTR), or a mixedjlow reactor (MFR). In this type of reactor, the composition in the reactor is assumed to be that of the effluent stream and therefore all the reaction occurs at this constant composition (Figure 3.3.1).
Since the reactor is at steady-state, the difference in F! (input) and Fi (output) must be due to the reaction. (In this text, the superscript 0 on flow rates denotes the input to the reactor.) The material balance on a CSTR is written as:
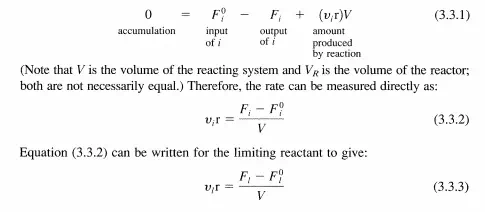

If VI = -1, then the reaction rate is equal to the number of moles of the limiting reactant fed to the reactor per unit time and per unit volume of the reacting fluid times the fractional conversion. For any product p not present in the feed stream, a material balance on p is easily obtained from Equation (3.3.1) with F? = 0 to give:
The quantity (FiV) is called the space-time yield. The equations provided above describe the operation of stirred-flow reactors whether the reaction occurs at constant volume or not. In these types of reactors, the fluid is generally a liquid. If a large amount of solvent is used, that is, dilute solutions of reactants/products, then changes in volume can be neglected. However, if the solution is concentrated or pure reactants are used (sometimes the case for polymerization reactions), then the volume will change with the extent of reaction.