Until the beginning of the 20th century, nitric acid was prepared commercially by reacting sulphuric acid with either potassium nitrate (saltpetre) or with sodium nitrate (Chile saltpetre or nitre). Up to four tonnes of the two ingredients were placed into large retorts and heated over a furnace. The volatile product vapourized and was collected for distillation.
An acid of between 93-95%(wt) was produced. In 1903 the electric-arc furnace superseded this primitive original technique. In the arc process, nitric acid was produced directly from nitrogen and oxygen by passing air through an electric-arc furnace.
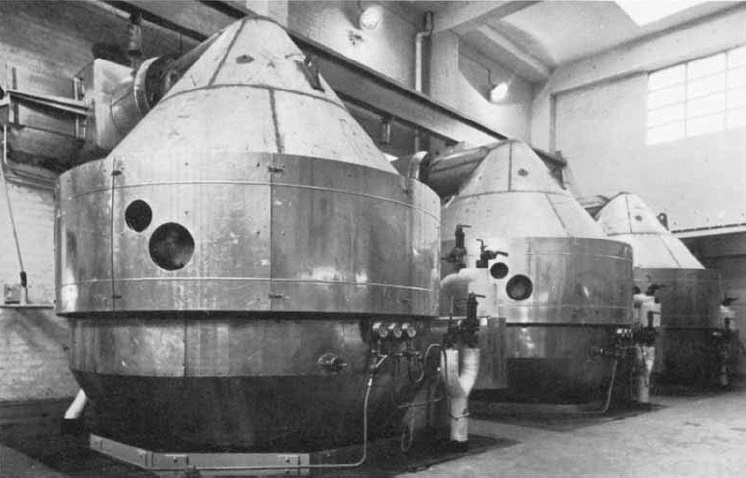
Although the process benefitted from an inexhaustible supply of free feed material (air), the power consumption for the arc furnace soon became cost prohibitive. Researchers returned to the oxidation of ammonia in air, (recorded as early as 1798) in an effort to improve production economics. In 1901 Wilhelm Ostwald had first achieved the catalytic oxidation of ammonia over a platinum catalyst. The gaseous nitrogen oxides produced could be easily cooled and dissolved in water to produce a solution of nitric acid. This achievement began the search for an economic process route. By 1908 the first commercial facility for production of nitric acid, using this new catalytic oxidation process, was commissioned near Bochum in Germany.
The Haber-Bosch ammonia synthesis process came into operation in 1913, leading to the continued development and assured future of the ammonia oxidation process for the production of nitric acid. During World War 1, the intense demand for explosives and synthetic dyestuffs created an expansion of the nitric acid industry. Many new plants were constructed, all of which employed the ammonia oxidation process. This increased demand served as the impetus for several breakthroughs in process technology. These included:
(a) The development of chrome-steel alloys for tower construction, replacing the heavy stoneware and acid-proof bricks. This enabled process pressures above atmospheric levels to be used.
(b) The improved design of feed preheaters enabled higher process temperatures to be attained. Higher temperatures improved the yields and capacities, and also reduced equipment requirements.
(c) Early developments in automatic process control improved process performance and reduced labour requirements. All of these factors helped to improve the process efficiency. The increasing availability of ammonia reduced processing costs still further.
In the late 1920’s the development of stainless steels enabled manufacturers to use higher operating pressures. The increase in yield and lower capital requirements easily justified the use of highpressure operation despite increased ammonia consumption. The introduction of higher pressure processes resulted in a divergence of operating technique within the industry. The United States producers opted for a high-pressure system, using a constanthigh pressure throughout the process. The European manufacturers opted for a split-pressure system. This latter system entails operating the ammonia oxidation section at atmospheric pressure, while the absorption unit is operated at higher pressures (capitalising on improved absorption rates). Recent developments in the ammonia oxidation process have included efforts to reduce catalyst losses in the process.
Platinum recovery filters have been installed at various stages in the process. Gold/palladium gauze filter pads have been added on the exit side of the catalyst bed, inside the reactor/converter units. These filters have reportedly ensured a platinum recovery of 80% (Ref. PT4).
Another trend has been for the use of additional filters in the downstream units. These filters are of alumino-silicate construction. Perhaps the greatest progress in nitric acid production technology has been in the manufacture of strong nitric acid ( >90% by weight). Advances in the areas of super-azeotropic distillation and in highpressure absorption are most significant. For further information consult References PT4 to PTl 1, PT14 and PT15.
Research work is continually being performed in an effort to reduce nitrogen oxide emissions from nitric acid plants. The Humphreys and Glasgow/Bolme nitric acid process is just one example of a new philosophy being applied to the absorption systems of weak nitric acid plants (50-68% by weight). Nitrogen oxide emissions have been reduced from 2000-5000 ppm to less than 1000 ppm (Ref. PT12, PT13). For the production of stronger nitric acid, tail gases are now being treated by selective or non-selective catalytic combustion systems. These innovative units have reduced the nitrogen oxide emissions to below 400 ppm (Ref. PT5, PT6).

Comments are closed