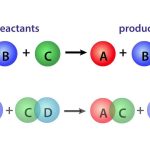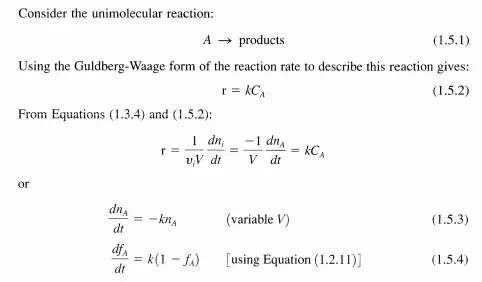
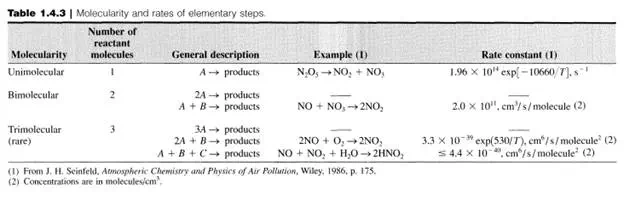
Thus, for first-order systems, the rate, r, is proportional (via k) to the amount present, ni’ in the system at any particular time. Although at first glance, firstorder reaction rates may appear too simple to describe real reactions, such is not the case (see Table 1.5.1). Additionally, first-order processes are many times used to approximate complex systems, for example, lumping groups of hydrocarbons into a generic hypothetical component so that phenomenological behavior can be described.