The changes in a chemically reacting system can frequently, but not always (e.g., complex fermentation reactions), be characterized by a stoichiometric equation. The stoichiometric equation for a simple reaction can be written as:

where NCOMP is the number of components, A;, of the system. The stoichiometric coefficients, Vi’ are positive for products, negative for reactants, and zero for inert components that do not participate in the reaction. For example, many gas-phase oxidation reactions use air as the oxidant and the dinitrogen in the air does not participate in the reaction (serves only as a diluent). In the case of ammonia synthesis the stoichiometric relationship is:
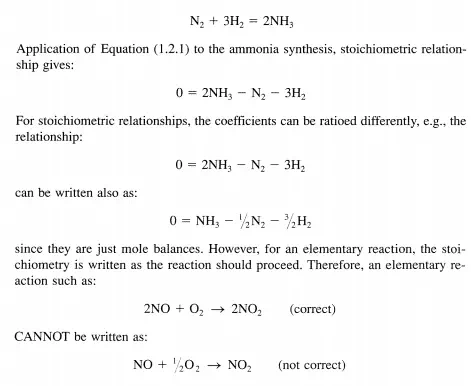
If there are several simultaneous reactions taking place, generalize Equation (1.2.1) to a system ofNRXN different reactions. For the methane oxidation network shown in Scheme 1.1.1, write out the relationships from the generalized equation.


