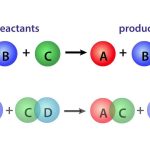
For a homogeneous, closed system at uniform pressure, temperature, and composition in which a single chemical reaction occurs and is represented by the stoichiometric Equation (1.2.1), the extent ofreaction as given in Equation (1.2.3) increases with time, t. For this situation, the reaction rate is defmed in the most general way by:
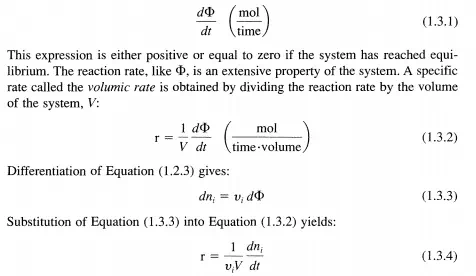
Since Vi is positive for products and negative for reactants and the reaction rate, d
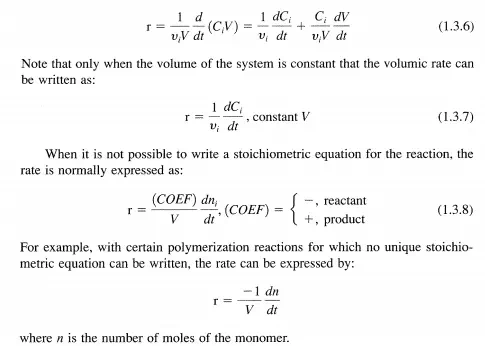
Thus far, the discussion of reaction rate has been confined to homogeneous reactions taking place in a closed system of uniform composition, temperature, and pressure. However, many reactions are heterogeneous; they occur at the interface between phases, for example, the interface between two fluid phases (gasliquid, liquid-liquid), the interface between a fluid and solid phase, and the interface between two solid phases. In order to obtain a convenient, specific rate of reaction it is necessary to normalize the reaction rate by the interfacial surface area available for the reaction. The interfacial area must be of uniform composition, temperature, and pressure. Frequently, the interfacial area is not known and alternative definitions of the specific rate are useful. Some examples of these types of rates are:
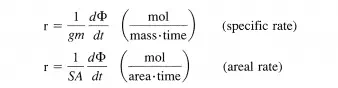
where gm and SA are the mass and surface area of a solid phase (catalyst), respectively. Of course, alternative definitions for specific rates of both homogeneous and heterogeneous reactions are conceivable. For example, numerous rates can be defined for enzymatic reactions, and the choice of the definition of the specific rate is usually adapted to the particular situation. For heterogeneous reactions involving fluid and solid phases, the areal rate is a good choice. However, the catalysts (solid phase) can have the same surface area but different concentrations of active sites (atomic configuration on the catalyst capable of catalyzing the reaction). Thus, a definition of the rate based on the number of active sites appears to be the best choice. The turnover frequency or rate of turnover is the number of times the catalytic cycle is completed (or turned-over) per catalytic site (active site) per time for a reaction at a given temperature, pressure, reactant ratio, and extent of reaction.