Enzymes are critical protein molecules in living systems that, once synthesized, are not usually transformed into some of other kind of molecule, as are the substances taken in as fuel for digestive and respiratory processes (e.g., sugars, fats, molecular oxygen). This is because enzymes are catalysts, which means that they can take part in chemical reactions without themselves being changed, a little like the moderator of a public debate who ideally moves the participants and the audience toward a conclusion by dictating the terms of the argument while not adding any unique information.
Over 2,000 enzymes have been identified, and each of them is involved with one specific chemical reaction. Enzymes are therefore substrate-specific. They are grouped into a half-dozen classes on the basis of the kinds of reactions they take part in.
Enzyme Basics
Enzymes permit a vast number of reactions to take place in the body under conditions of homeostasis, or overall biochemical balance. For example, many enzymes function best at a pH (acidity) level close to the pH the body normally maintains, which is in the range of 7 (that is, neither alkaline nor acidic). Other enzymes function best at low pH (high acidity) because of the demands of their environment; for example, the inside of the stomach, where some digestive enzymes operate, is highly acidic.
Enzymes take part in processes ranging from blood clotting to DNA synthesis to digestion. Some are found only within cells and participate in processes involving small molecules, such as glycolysis; others are secreted directly into the gut and act on bulk matter such as swallowed food. Because enzymes are proteins with fairly high molecular masses, they each have a distinct three-dimensional shape. This determines the specific molecules on which they act. In addition to being pH-dependent, the shape of most enzymes is temperature-dependent, meaning that they function best in a fairly narrow temperature range.
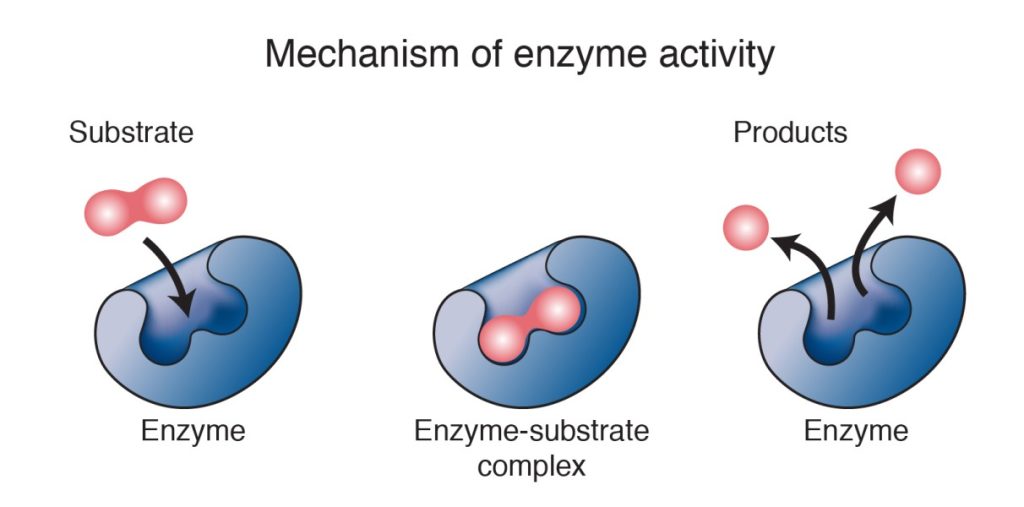
How Enzymes Work
Most enzymes work by lowering the activation energy of a chemical reaction. Sometimes, their shape brings the reactants physically close together in the style, perhaps, of a sports-team coach or work-group manager intent on getting a task done more quickly. It is believed that when enzymes bind to a reactant, their shape changes in a way that destabilizes the reactant and makes it more susceptible to whatever chemical changes the reaction involves.
Reactions that can proceed without the input of energy are called exothermic reactions. In these reactions, the products, or the chemical(s) formed during the reaction, have a lower energy level than the chemicals that serve as the reaction’s ingredients. In this way, molecules, like water, “seek” their own (energy) level; atoms “prefer” to be in arrangements with lower total energy, just like water flows downhill to the lowest available physical point. Putting all of this together, it is clear that exothermic reactions always proceed naturally.
However, the fact that a reaction will occur even without input says nothing about the rate at which it will happen. If a substance taken into the body will naturally change into two derivative substances that can serve as direct sources of cellular energy, this does little good if the reaction naturally takes hours or days to complete. Also, even when the total energy of products is higher than that of the reactants, the energy path is not a smooth downhill slope on a graph; instead, the products must attain a higher level of energy than that with which they began so that they can “get over the hump” and the reaction may proceed. This initial investment of energy into the reactants that pays off in the form of products is the aforementioned energy of activation, or Ea.
Types of Enzymes
The human body includes six major groups, or classes, of enzymes.
Oxidoreductases enhance the rate of oxidation and reduction reactions. In these reactions, also called redox reactions, one of the reactants gives up a pair of electrons that another reactant gains. The electron-pair donor is said to be oxidized and acts as a reducing agent, while the electron-pair recipient is reduced is called the oxidizing agent. A more straightforward way of putting this is that in these kinds of reactions, oxygen atoms, hydrogen atoms or both are moved. Examples include cytochrome oxidase and lactate dehydrogenase.
Transferases speed along the transfer of groups of atoms, such as methyl (CH3), acetyl (CH3CO) or amino (NH2) groups, from one molecule to another molecule. Acetate kinase and alanine deaminase are examples of transferases.
Hydrolases accelerate hydrolysis reactions. Hydrolysis reactions use water (H2O) to split a bond in a molecule to create two daughter products, usually by affixing the -OH (hydroxyl group) from the water to one of the products and a single -H (hydrogen atom) to the other. In the meantime, a new molecule is formed from the atoms displaced by the -H and -OH components. The digestive enzymes lipase and sucrase are hydrolases.
Lyases enhance the rate of the addition of one molecular group to a double bond or the removal of two groups from nearby atoms to create a double bond. These act like hydrolases, except that the removed component is not displaced by water or portions of water. This class of enzymes includes oxalate decarboxylase and isocitrate lyase.
Isomerases speed up isomerization reactions. These are reactions in which all of the original atoms in the reactant are retained, but are rearranged to form an isomer of the reactant. (Isomers are molecules with the same chemical formula, but different arrangements.) Examples include glucose-phosphate isomerase and alanine racemase.
Ligases (also called synthetases) enhance the rate of the joining of two molecules. They usually accomplish this by making use of energy derived from the breakdown of adenosine triphosphate (ATP). Examples of ligases include acetyl-CoA synthetase and DNA ligase.
Enzyme Inhibition
In addition to temperature and pH changes, other factors can result in an enzyme’s activity being diminished or shut down. In a process called an allosteric interaction, the shape of the enzyme is temporarily changed when a molecule binds to a portion of it away from where it joins the reactant. This leads to a loss of function. Sometimes this is useful when the product itself serves as the allosteric inhibitor, because this is usually a sign of the reaction having proceeded to the point where additional product is no longer required.
In competitive inhibition, a substance called a regulatory compound competes with the reactant for the binding site. This is akin to trying to put several working keys into the same lock at the same time. If enough of these regulatory compounds join to a high enough amount of the enzyme present, it slows or shuts down the reaction pathway. This can be helpful in pharmacology because microbiologists can design compounds that compete with the binding sites of bacterial enzymes, making it much harder for the bacteria to cause disease or survive in the human body, period.
In noncompetitive inhibition, an inhibitory molecule binds to the enzyme at a spot different from the active site, similar to what happens in an allosteric interaction. Irreversible inhibition occurs when the inhibitor permanently binds to or significantly degrades the enzyme so that its function cannot recover. Nerve gas and penicillin both make use of this type of inhibition, albeit with massively different intentions in mind.

Comments are closed