What Are Enzymes?
Enzymes are proteins that act as catalysts within living cells. Catalysts increase the rate at which chemical reactions occur without being consumed or permanently altered themselves. A chemical reaction is a process that converts one or more substances (known as reagents, reactants, or substrates) to another type of substance (the product). As a catalyst, an enzyme can facilitate the same chemical reaction over and over again.
Structure and Function
Like all proteins, enzymes are composed of one or more long chains of interconnected amino acids. Each enzyme possesses a unique sequence of amino acids that causes it to fold into a characteristic shape. An enzyme’s amino acid sequence is determined by a specific gene in the cell’s nucleus. This ensures that each copy of the enzyme is the same as all others.
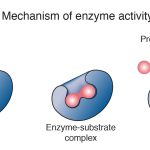
On the surface of each enzyme is a special cleft called the active site, which provides a place where reagents can ‘meet’ and interact. Much like a lock and its key, an enzyme’s active site will only accommodate certain reagents, and only one type of chemical reaction can be catalyzed by a given enzyme.
For example, during the manufacture of hemoglobin (the oxygen-carrying pigment in your red blood cells), a single atom of iron must be inserted into the center of the molecule to make it functional. An enzyme called ferrochelatase brings the reagents (iron and the empty molecule) together, catalyzes their union, and releases an iron-containing molecule. This is the only reaction catalyzed by ferrochelatase. Keep in mind that enzymes can combine reagents (as in the synthesis of hemoglobin), they can split a single reagent into multiple products, or they can simply transform a single reagent into a single product that looks different from the original reagent.
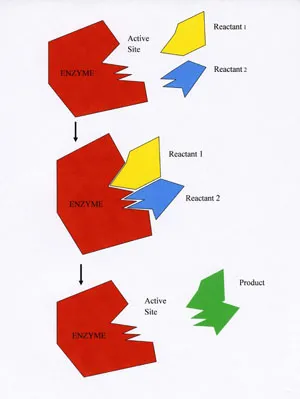 |
When reagents enter an enzyme’s active site, the enzyme undergoes a temporary change in shape that encourages interaction between the reagents. Upon completion of the chemical reaction, a specific product is released from the active site, the enzyme resumes its original conformation, and the reaction can begin again with new reagents.
Many enzymes are incorporated into metabolic pathways. A metabolic pathway is a series of chemical reactions that transform one or more reagents into an end-product that’s needed by the cell. The enzymes in a metabolic pathway–much like people passing a pail of water along a bucket brigade–move a reagent along until the end-product is produced. A metabolic pathway can be quite short, or it can have many steps and multiple enzymes. The metabolic pathway that converts tryptophan (an amino acid found in dietary protein) to serotonin (a chemical that’s necessary for normal brain function) is only two steps long.
In order to function, many enzymes require the help of cofactors or coenzymes. Cofactors are often metal ions, such as zinc, copper, iron, or magnesium. Magnesium, one of the most common cofactors, activates hundreds of enzymes, including those that manufacture DNA and many that help metabolize carbohydrates.
Many coenzymes are derived from vitamins. In fact, one of the main reasons you need vitamins in your diet is to supply the raw material for essential coenzymes. For example, vitamin C is needed by the enzyme that produces collagen and builds healthy skin, a coenzyme derived from vitamin B12 is necessary for synthesizing the insulation around your nerve cells, and a vitamin B6-based coenzyme is vital for producing serotonin.
Coenzymes and cofactors bind to the active sites of enzymes, and they participate in catalysis, but they are not generally considered reagents, nor do they become part of the product(s) of the reaction. In many cases, cofactors and coenzymes function as intermediate carriers of electrons, specific atoms, or functional groups that are transferred during the overall reaction.
Why Do We Need Enzymes?
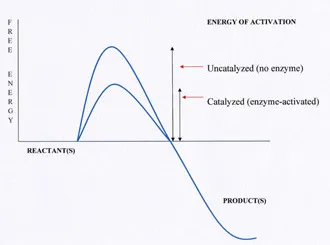
One of the immutable laws of nature is that energy is required to initiate a chemical reaction. After all, if you want to change something, you have to put some work into it. For every chemical reaction, the energy of activation–a specific amount of energy–must be applied to the reagents before the reaction will proceed. The energy of activation is like a hill between the reagents and the product, and the reagents must be pushed over this hill before the reaction can continue.
 |
Comments are closed