What IS an ideal gas?
Recall from general chemistry that the volume, pressure, temperature, and moles of a gas in a closed system can be related by the following equation, which is referred to as the ideal gas law:

Theoretical background on the ideal gas law
The ideal gas law relies on several rather strong assumptions about the nature of gasses, which make up the classical Kinetic Theory of Gases. These assumptions are:
1. That gas molecules do not interact with each other whatsoever.
2. That all collisions between molecules and each other or with the walls of the container are completely elastic, meaning the mean kinetic energy of the molecules stays the same.
3. That gas molecules are very small compared to the distance between them.
There are several other assumptions as well, but these will suffice to explain why deviations occur.
Important facts about ideal gasses[
Ideal gasses are nice because they have several properties that make them relatively easy and useful to work with:
1. A mixture of ideal gasses is also an ideal gas. Therefore, you can use the ideal gas law on the entire mixture or on any of its components without loss of validity.
2. The Partial Pressure of a component in an ideal gas mixture is related to the total pressure by the equation  where
where  is the mole fraction of component A in the mixture.
is the mole fraction of component A in the mixture.
3. The ideal gas equation, if it is valid, is independent of the properties of the gas. Therefore, there is no need to look up or measure gas-specific parameters, all you need to know is 3 of the unknowns (P,V,T, and n) and you can solve for the fourth one.
4. The enthalpy and entropy of an ideal gas only depend on temperature (not pressure or volume).
5. Many gasses are close to ideal at low pressures and high temperatures. Therefore it can be used as a realistic reference state to which a real gas can be compared.
There are many other useful properties of ideal gasses that will be discussed in thermodynamics.
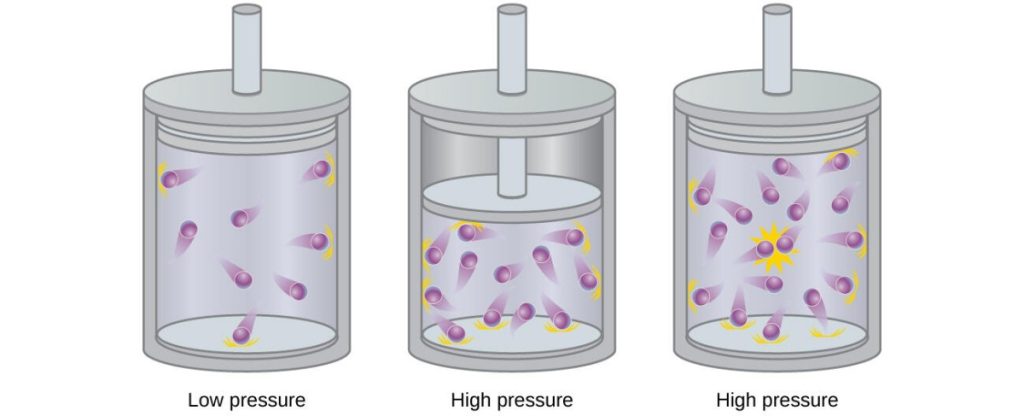
Deviations at high pressure
Suppose that you have water vapor in a small water bottle. What happens when you apply pressure to that vapor by shrinking the volume? If you apply more pressure to the bottle, the gas molecules within become closer and closer together. However, the closer molecules of a substance are to one another, the larger the dispersion forces (and polar forces, if applicable) are between them. Eventually, the dispersion forces become significant, and the kinetic theory of gasses as stated above is no longer valid. Therefore, the ideal gas law no longer applies, or becomes a rough estimate at best.
At very high pressures, a vapor might even condense into a liquid, which would also invalidate the use of the law.
Deviations at low temperature
By definition of temperature, when the temperature of a substance decreases, this indicates a lower average kinetic energy of the molecules of that substance. Molecules with less kinetic energy also have less momentum, and therefore are more easily swayed by dispersion forces from other molecules and by gravity. Eventually, the temperature might become low enough that the forces from other molecules cause significant deviations in the path of a molecule, and in this case the ideal gas law becomes less valid.
As with pressure, a very low temperature can cause a gas to condense.
Rule of thumb for use of the ideal gas law
Due to the above two discussions, we can claim that the ideal gas law is a decent assumption at high temperatures and low pressures, but should not be used outside that realm.
The Idea of Equations of State
The ideal gas law is not the only way to express relationships between system properties. There are an infinite number of possible ways that you could propose to correlate system variables, although thermodynamics provides some guidelines regarding how many variables to correlate and what types of correlations we should look for. In particular, it is most useful to correlate relationships between state variables, for which changes in the properties do not depend on how the change occurred.
Any equation that relates state variables is called an equation of state. The most commonly-used equations of state relate the variables P, T, V, and n (pressure, temperature, volume, and number of moles) since they are all measurable variables whereas many other possible variables are not directly measurable (such as enthalpy, which will be discussed later).
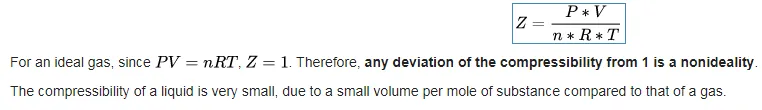
Compressibility
There is a highly useful quantity for describing how much a gaseous system deviates from ideality, which is called the compressibility of the gas. The compressibility Z is defined as:
Ideal Gas Law Extension
One use of the compressibility is that it allows a simple extension of the ideal gas law, which is completely general.

Unfortunately, Z is not a constant for any material, but changes with pressure and temperature. However, in a later section you will learn a technique called the generalized compressibility method with which you can estimate the value of Z for any substance, given certain data. Once it is known, this extension can be used to calculate an unknown system property.
Alternatives to the ideal gas law 1: Van der Waals Equation
One of the oldest equations that was developed to take non-ideality into account is called the Van der Waals Equation, which has two substance-dependent parameters. One takes into account the interactions between particles, and the other the fact that particles have volume, sometimes substantial.
The Van der Waals equation is as follows:
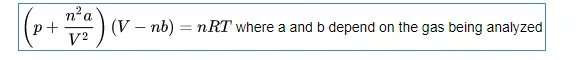
A list of values for a and b can be found on Wikipedia at this page.
The Van der Waals equation is significantly more accurate than the ideal gas law and can be used to crudely predict when a gas will condense. However, it is not sufficiently accurate for many industrial purposes, and therefore other methods have been sought since then.
Alternatives to the ideal gas law 2: Virial Equation
The Virial equation is an equation which has a potentially infinite number of parameters that depend on the properties of the substances involved. It is important because it can be shown to be a valid extension to the ideal gas law using statistical theories (whereas the other equations of state have been derived semi-empirically).
The virial equation can take several forms, depending on the data one has available. The one in terms of molar volume is:
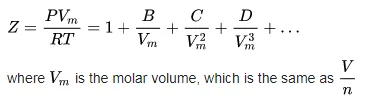
Alternatives to the ideal gas law 3: Peng-Robinson equation
One of the more modern equations of state is the Peng-Robinson equation, which is most useful for describing nonpolar molecules such as hydrocarbons or nitrogen. The Peng-Robinson equation has two parameters like the Van der Waals equation, but unlike the latter, one of the parameters is not constant; it depends on the temperature of the system as well as the properties of the substance inside.