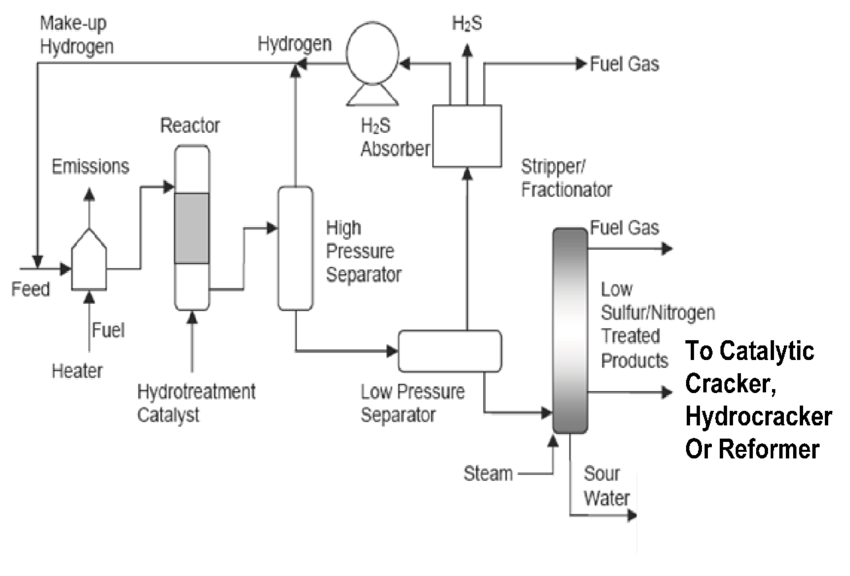
The objective of the Hydrotreating prococess is to remove suplur as well as other unwanted compunds, e.g. unsaturated hydrocarbons, nitrogen from refinery process streams.
Until the end of World War 2, there was little incentive for the oil industry to pay significant attention to improving product quality by hydrogen treatment. However, soon after the war the production of high sulphur crudes increased significantly, which gave a more stringent demand on the product blending flexibility of refineries, and the marketing specifications for the products became tighter, largely due to environmental considerations. Furthermore, the catalyst used in the Platforming process can only handle sulfur in the very low ppm level, so hydrotreating of naphtha became a must. The necessity for hydrotreating of middle distillates (kerosene/gasoil) originates from pressure to reduce sulfur emissions into the environment. Overall, this situation resulted in an increased necessity for high sulphur removal capability in many refineries.
As catalytic reforming gives hydrogen as a byproduct, it gave additional momentum to the development of sulphur removal process by hydrogen treatment. In this treatment, the sulphur compounds are removed by converting them into hydrogen sulphide by reaction with hydrogen in the presence of a catalyst. This results in high liquid product yields, since only sulphur is removed. Furthermore, the hydrogen sulphide produced can be easily removed from the product gas stream, for example by an amine wash. In this way, hydrogen sulphide is recovered as a higly concentrated stream and can be further converted into elemental sulphur via the “Claus” process.
Hydrodesulphursiation has been extensively used commercially for treating naphtha as feedstock for catalytic reformers to meet the very stringent sulphuir specification of less than 1 ppm wt to protect the platinum catalyst. It has also been widely used for removal of sulphur compounds from kerosine and gasoils to make them suitable as blending components. In cases where products are from catalytic or thermal crackers, hydrogen treatment is used to improve product quality specifications like colour, smoke point, cetane index, etc.
For Hydrotreating, two basic processes are applied, the liquid phase (or trickle flow) process for kerosine and heavier straight-run and cracked distillates up to vacumn gas oil and the vapour phase process for light straight-run and cracked fractions.Both processes use the same basic configuration: the feedstock is mixed with hydrogen-rich make up gas and recycle gas. The mixture is heated by heat exchange with reactor effluent and by a furnace and enters a reactor loaded with catalyst. In the reactor, the sulphur and nitrogen compounds present in the feedstock are converted into hydrogen sulphide and ammonia respectively. The olefins present are saturated with hydrogen to become di-olefins and part of the aromatics will be hydrogenated. If all aromatics needs to be hydrogenated, a higher pressure is needed in the reactor compared to the conventional operating mode.
The reactor operates at temperatures in the range of 300-380 0C and at a pressure of 10-20 bar for naphta and kero, as compared with 30-50 bar for gasoil, with excess hydrogen supplied. The temperature should not exceed 380 0C, as above this temperature cracking reactions can occur, which deteriorates the colour of the final product. The reaction products leave the reactor and, after having been cooled to a low temperature, typically 40-50 0C, enter a liquid/gas separation stage. The hydrogen-rich gas from the high pressure separation is recycled to combine with the feedstock, and the low pressure off-gas stream rich in hydrogen sulphide is sent to a gas-treating unit, where hydrogen sulphide is removed. The clean gas is then suitable as fuel for the refinery furnaces. The liquid stream is the product from hydotreating. It is normally sent to a stripping column where H2S and other undesirable components are removed, and finally, in cases where steam is used for stripping, the product is sent to a vacumn drier for removal of water. Some refiners use a salt dryer in stead of a vacuum drier to remove the water.
The catalyst used is normally cobalt, molybdenum and nickel finely distributed on alumina extrudates. It slowly becomes choked by coke and must be renewed at regular intervals (typically 2-3 years). It can be regenerated (by burning off the coke) and reused typically once or twice before the breakdown of the support”s porous structure unacceptably reduces its activity. Catayst regeneration is, nowadays, mainly carried out ex- situ by specialised firms. Other catalysts have also been developed for applications where denitrification is the predominant reaction required or where high stauration of olefins is necessary.
Description: Photo of a Refinery
Figure 3: Photo of a Typical Refinery
A more recent development is the application of Hydrotreating for pretreatment of feedstcok for the catalytic cracking process. By utilisation of a suitable hydrogenation-promoting catalyst for conversion of aromatics and nitrogen in potential feedstocks, and selection of severe operating conditions, hydrogen is taken up by the aromatic molecules. The increased hydrogen content of the feedstock obtained by this treatment leads to significant conversion advantages in subsequent catalytic cracking, and higher yield of light products can be achieved.
Hydrotreatment can also be used for kerosine smoke point improvement (SPI). It closely resembles the conventional Hydrotreating Process however an aromatic hydrogenation catalyst consisting of noble metals on a special carrier is used. The reactor operates at pressure range of 50-70 bar and temperatures of 260-320Â 0C. To restrict temperature rise due to the highly exothermic aromatics conversion reactions, quench oil is applied between the catalysts beds. The catalyst used is very sensitive to traces of sulphur and nitrogen in the feedstock and therefore pretreatment is normally applied in a conventional hydrotreater before kerosine is introduced into the SPI unit. The main objective of Smoke Point Improvement is improvement in burning characteristics as the kerosine aromatics are converted to naphthenes.
Hydrotreatment is also used for production of feedstocks for isomersiation unit from pyrolysis gasoline (pygas) which is one of the byproducts of steam cracking of hydrocarbon fractions such as naphtha and gasoil.
A hyrotreater and a hydrodesulphuriser are basically the same process but a hydotreater termed is used for treating kerosene or lighter feedstock, while a hydodesulhuriser mainly refers to gasoil treating. The hydrotreatment process is used in every major refinery and is therefore also termed as the work horse of the refinery as it is the hydrotreater unit that ensures several significant product quality specifications. In most countries the Diesel produced is hydrodesulhurised before its sold. Sulphur specifications are getting more and more stringent. In Asia, countries such as Thailand, Singapore and Hong Kong already have a 0.05%S specification and large hydrodesulphurisation units are required to meet such specs.
The by-products obtained from HDT/HDS are light ends formed from a small amounts of cracking and these products are used in the refinery fuelgas pool. The other main by-product is Hydrogen Sulphide which is oxidized to sulphur and sold to the chemical industry for further processing In combination with temperature, the pressure level (or rather the partial pressure of hydrogen) generally determines the types of components that can be removed and also determines the working life of the catalyst. At higher (partial) pressures, the desulphurisation process is ”easier”, however, the unit becomes more expensive for instance due to larger compressors and heavier reactors. Also, at higher pressure, the hydrogen consumption of the unit increases, which can be a signficant cost factor for the refinery. The minimum pressure required typically goes up with the required severity of the unit, i.e. the heavier the feedstock, or the lower levels of sulphur in product required.