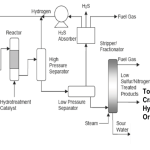
Motor gasoline (Mogas) production starts with the distillation of crude oil. One of the products out of that process is a fraction of low octane gasoline, normally referred to as naphtha, typically boiling in the range 100 – 160 °C. Other gasoline fractions are produced as a result of secondary processes like catalytic cracking, isomerisation, alkylation and platforming. Petrol is then produced by blending a variety of these gasoline components of different qualities to meet a series of product specifications.
One very important property of Mogas is the octane number, which influences “knocking” or “pinking” behaviour in the engine of cars.
Traditionally lead compounds have been added to petrol to improve the octane number. Over the past years, in many countries legislation has been implemented aimed at reducing the emission of lead from exhausts of motor vechiles and this, calls for other means of raising the octane number.
The role of a platformer is to pave the way for this by a process which reforms the molecules in low octane naphtha to produce a high octane gasoline component. This is achieved by employing a catalyst with platinum as its active compound; hence the name Platformer. For many refinery catalyst applications, a promoter is used, and in the platforming process, it is a chloride promoter which stimulates the ”acidity” of the catalyst and thereby the isomerisation reactions. Often, a bimetallic catalyst is used, i.e. in addition to the platinum, a second metal, for instance Rhenium is present on the catalyst. The main advantage is a higher stability under reforming conditions. The disadvantage is that the catalyst becomes more sensitive towards poisons, process upsets and more susceptible to non-optimum regenerations.