Broadband-light sources
Although flames and discharges provide a convenient method of excitation, the environment can strongly perturb the sample being studied. Excitation based on broadband-light sources in which the generation of the light is separated from the sample to be investigated provides a less perturbing means of excitation. Higher energy excitation corresponds to shorter wavelengths, but unfortunately, there are not many intense sources of ultraviolet and vacuum-ultraviolet radiation, and so excitation in an electron discharge remains a common method for this portion of the spectrum. (The term vacuum ultraviolet refers to the short-wavelength portion of the electromagnetic spectrum where the photons are energetic enough to excite a typical atom from the ground state to ionization. Under these conditions, the light is strongly absorbed by air and most other substances.)
A typical broadband-light source that can be used for either emission or absorption spectroscopy is a metal filament heated to a high temperature. A typical example is a tungsten lightbulb. Because the atoms in the metal are packed closely together, their individual energy levels merge together; the emitted lines then overlap and form a continuous—i.e., nondiscrete—spectrum. Similar phenomena occur in high-pressure arc lamps, in which broadening of spectral lines occurs owing to high collision rates.
An arc lamp consists of a transparent tube of gases that are excited by an electric discharge. Energetic electrons bombard the atoms, exciting them to either high-energy atomic states or to an ionized state in which the outermost electron is removed from the atom. The radiation that is emitted in this environment is usually a mixture of discrete atomic lines that come from the relaxation of the atoms to lower energy states and continuum radiation resulting from closely spaced lines that have been broadened by collisions with other atoms and the electrons. If the pressure of the gas in the arc lamp is sufficiently high, a large fraction of the light is emitted in the form of continuum radiation.
Line sources
Light sources that are capable of primarily emitting radiation with discrete, well-defined frequencies are also widely used in spectroscopy. The early sources of spectral emission lines were simply arc lamps or some other form of electrical discharge in a sealed tube of gas in which the pressure is kept low enough so that a significant portion of the radiation is emitted in the form of discrete lines. The Geissler discharge tube, such as the neon lamp commonly used in advertising signs, is an example of such a source. Other examples are hollow cathode lamps and electrodeless lamps driven by microwave radiation. If specific atomic lines are desired, a small amount of the desired element is introduced in the discharge.
Laser sources
Lasers are line sources that emit high-intensity radiation over a very narrow frequency range. The invention of the laser by the American physicists Arthur Schawlow and Charles Townes in 1958, the demonstration of the first practical laser by the American physicist Theodore Maiman in 1960, and the subsequent development of laser spectroscopy techniques by a number of researchers revolutionized a field that had previously seen most of its conceptual developments before the 20th century. Intense, tunable (adjustable-wavelength) light sources now span most of the visible, near-infrared, and near-ultraviolet portions of the spectrum. Lasers have been used for selected wavelength bands in the infrared to submillimetre range, and on the opposite end of the spectrum, for wavelengths as short as the soft X-ray region (that of lower energies).
Typically, light from a tunable laser (examples include dye lasers, semiconductor diode lasers, or free-electron lasers) is directed into the sample to be studied just as the more traditional light sources are used in absorption or emission spectroscopy. For example, in emission (fluorescence) spectroscopy, the amount of light scattered by the sample is measured as the frequency of the laser light is varied. There are advantages to using a laser light source: (1) The light from lasers can be made highly monochromatic (light of essentially one “colour”—i.e., composed of a very narrow range of frequencies). As the light is tuned across the frequency range of interest and the absorption or fluorescence is recorded, extremely narrow spectral features can be measured. Modern tunable lasers can easily resolve spectral features less than 106 hertz wide, while the highest-resolution grating spectrometers have resolutions that are hundreds of times lower. Atomic lines as narrow as 30 hertz out of a transition frequency of 6 × 1014 hertz have been observed with laser spectroscopy. (2) Because the laser light in a given narrow frequency band is much more intense than virtually all broadband sources of light used in spectroscopy, the amount of fluorescent light emitted by the sample can be greatly increased. Laser spectroscopy is sufficiently sensitive to observe fluorescence from a single atom in the presence of 1020 different atoms.
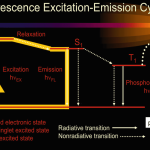
A potential limitation to the resolution of the spectroscopy of gases is due to the motion of the atoms or molecules relative to the observer. The Doppler shifts that result from the motion of the atoms will broaden any sharp spectral features. A cell containing a gas of atoms will have atoms moving both toward and away from the light source, so that the absorbing frequencies of some of the atoms will be shifted up while others will be shifted down. The spectra of an absorption line in the hydrogen atom as measured by normal fluorescence spectroscopy is shown in Figure 1A. The width of the spectral features is due to the Doppler broadening on the atoms
Energy states of real diatomic molecules
For any real molecule, absolute separation of the different motions is seldom encountered since molecules are simultaneously undergoing rotation and vibration. The rigid-rotor, harmonic oscillator model exhibits a combined rotational-vibrational energy level satisfying EvJ = (v + 1/2)hν0 + BJ(J + 1). Chemical bonds are neither rigid nor perfect harmonic oscillators, however, and all molecules in a given collection do not possess identical rotational, vibrational, and electronic energies but will be distributed among the available energy states in accordance with the principle known as the Boltzmann distribution.
As a molecule undergoes vibrational motion, the bond length will oscillate about an average internuclear separation. If the oscillation is harmonic, this average value will not change as the vibrational state of the molecule changes; however, for real molecules the oscillations are anharmonic. The potential for the oscillation of a molecule is the electronic energy plotted as a function of internuclear separation (Figure 7A). Because this curve is nonparabolic, the oscillations are anharmonic and the energy levels are perturbed. This results in a decreasing energy level separation with increasing v and a modification of the vibrational selection rules to allow Δv = ±2, ±3,….
Since the moment of inertia depends on the internuclear separation by the relationship I = μr2, each different vibrational state will possess a different value of I and therefore will exhibit a different rotational spectrum. The nonrigidity of the chemical bond in the molecule as it goes to higher rotational states leads to centrifugal distortion; in diatomic molecules this results in the stretching of the bonds, which increases the moment of inertia. The total of these effects can be expressed in the form of an expanded energy expression for the rotational-vibrational energy of the diatomic molecule.
A molecule in a given electronic state will simultaneously possess discrete amounts of rotational and vibrational energies. For a collection of molecules they will be spread out into a large number of rotational and vibrational energy states so any electronic state change (electronic transition) will be accompanied by changes in both rotational and vibrational energies in accordance with the proper selection rules. Thus any observed electronic transition will consist of a large number of closely spaced members owing to the vibrational and rotational energy changes.
Experimental methods
There are three basic types of spectrometer systems that are commonly used for molecular spectroscopy: emission, monochromatic radiation absorption, and Fourier transform. Each of these methods involves a source of radiation, a sample, and a device for detecting and analyzing radiation.
Emission spectrographs have some suitable means of exciting molecules to higher energy states. The radiation emitted when the molecules decay back to the original energy states is then analyzed by means of a monochromator and a suitable detector. This system is used extensively for the observation of electronic spectra. The electrons are excited to higher levels by means of an energy source such as an electric discharge or a microwave plasma. The emitted radiation generally lies in the visible or ultraviolet region. Absorption spectrometers employ as sources either broadband radiation emitters followed by a monochromator to provide a signal of very narrow frequency content or a generator that will produce a tunable single frequency. The tunable monochromatic source signal then passes through a sample contained in a suitable cell and onto a detector designed to sense the source frequency being used. The resulting spectrum is a plot of intensity of absorption versus frequency.
A Fourier-transform spectrometer provides a conventional absorption spectrometer-type spectrum but has greater speed, resolution, and sensitivity. In this spectrometer the sample is subjected to a broadband source of radiation, resulting in the production of an interferogram due to the absorption of specific components of the radiation. This interferogram (a function of signal intensity versus time) is normally digitized, stored in computer memory, and converted to an absorption spectrum by means of a Fourier transform . Fourier-transform spectrometers can be designed to cover all spectral regions from the radio frequency to the X-ray.
Spectrometers allow the study of a large variety of samples over a wide range of frequencies. Materials can be studied in the solid, liquid, or gas phase either in a pure form or in mixtures. Various designs allow the study of spectra as a function of temperature, pressure, and external magnetic and electric fields. Spectra of molecular fragments obtained by radiation of materials and of short-lived reaction intermediates are routinely observed. Two useful ways to observe spectra of short-lived species at low (4 K) temperature are to trap them in a rare gas matrix or to produce them in a pulsed adiabatic nozzle.

Comments are closed