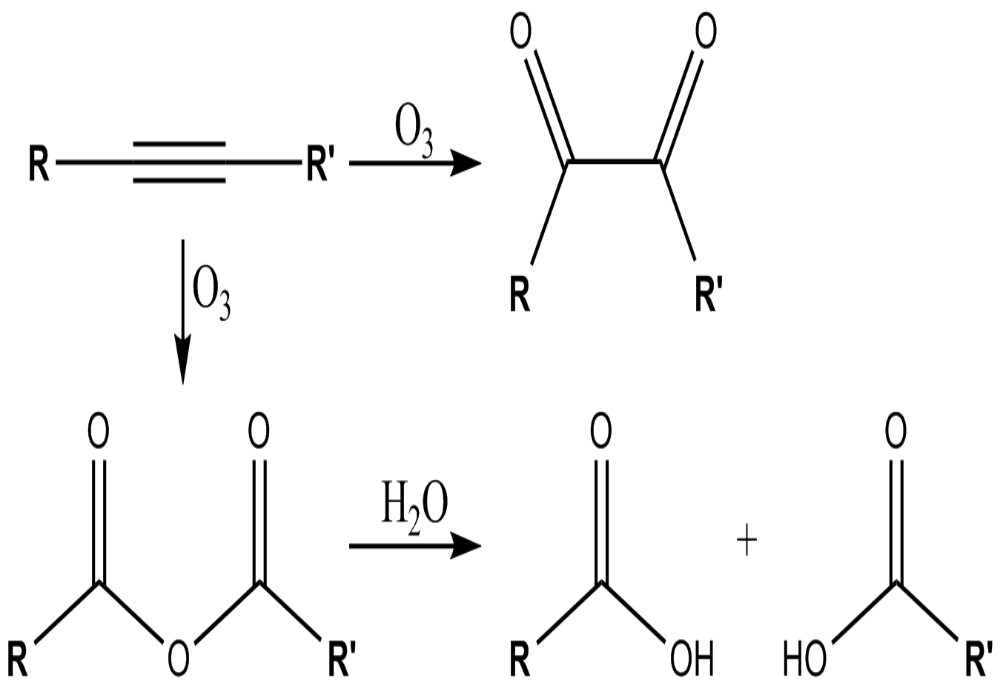
Alkynes are unsaturated hydrocarbons which consist of at least one triple bond between carbon atoms. There are two types of alkynes: terminal and internal. Terminal alkynes are triple bonded compounds in which a carbon atom shares the triple bond with the carbon at the end of the chain. Internal alkynes are compounds in which the triple bond is between two carbon atoms, none of which are terminal. The general molecular formula of alkynes is CnH2n-2. Physical properties of alkynes are very similar to the physical properties of alkenes.
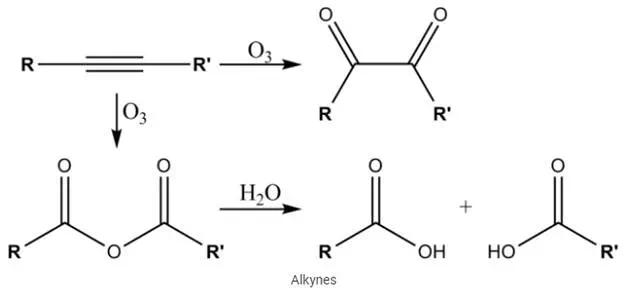
The uniqueness in the alkyne structure is due to the hybridization. The acidity of alkynes, non-polar bonding strength, and linearity is due to the triple bonds in these compounds. These compounds are slightly soluble in polar solvents and are totally insoluble in water. Alkynes have the capability of dissolving in organic solvents as the density of the solution is less, which is a characteristic feature of alkenes as well. For example: it has the capability to dissolve in ether solution.
These triple bonded compounds have a boiling point slightly higher than alkanes and alkenes. For example: The boiling point of Ethane is -88.6 C while Ethene is -103.7 C and Ethyne has a boiling point of -84 C. The boiling point increases with the increase in number of carbon atoms.
The acidity of these triple bonded compounds is greater than its counterparts i.e. alkanes and alkenes. The sp hybridized alkynes are most acidic. It can be deprotonated only by using strong bases. For example ethane has a pKa vale of 62 which makes it least acidic, while ethene has a pKa value of 45 and ethyne is the most acidic with a pKa value of 26.